The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer
- PMID: 20926342
- PMCID: PMC2981619
- DOI: 10.1016/j.coph.2010.09.007
The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer
Abstract
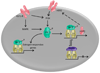
Our understanding of the molecular mechanisms underlying the pharmacological actions of estrogen receptor (ER) ligands has evolved considerably in recent years. Much of this knowledge has come from a detailed dissection of the mechanism(s) of action of the Selective Estrogen Receptor Modulators (SERMs) tamoxifen and raloxifene, so called for their ability to function as ER agonists or antagonists depending on the tissue in which they operate. These mechanistic insights have had a significant impact on the discovery of second generation SERMs, some of which are in late stage clinical development for the treatment/prevention of breast cancer as well as other estrogenopathies. In addition to the SERMs, however, have emerged the Selective Estrogen Degraders (SERDs), which as their name suggests, interact with and facilitate ER turnover in cells. One drug of this class, fulvestrant, has been approved as a third line treatment for ER-positive metastatic breast cancer. Whereas the first generation SERMs/SERDs were discovered in a serendipitous manner, this review will highlight how our understanding of the molecular pharmacology of ER ligands has been utilized in the development of the next generation of SERMs/SERDs, some of which are likely to have a major impact on the pharmacotherapy of breast cancer.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

References
-
- Clemens JA, Bennet DR, Black LJ, Jones CD. Effects of a new anti-estrogen, keoxifene (LY156758), on growth of carcinogen-induced mammary tumors and on LH and prolactin secretion. Life Sciences. 1983;32:2869–2875. - PubMed
-
- Jordan VC, Collins MM, Rowsby L, Prestwich G. A mono-hydroxylated metabolite of tamoxifen with potent antioestrogen activity. J Endocrinol. 1977;75:305–316. - PubMed
-
- Harper MJK, Walpole AL. Contrasting endocrine activities of cis and trans isomers in a series of substituted triphenylethylenes. Nature. 1966;212:87–89. - PubMed
-
- Turner CH, Sato M, Bryant HU. Raloxifene preserves bone strength and bone mass in ovariectomized rats. Endocrinology. 1994;135:2001–2005. - PubMed
-
- Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Min Res. 1987;2:449–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

