Sleep and synaptic renormalization: a computational study
- PMID: 20926617
- PMCID: PMC3007640
- DOI: 10.1152/jn.00593.2010
Sleep and synaptic renormalization: a computational study
Abstract
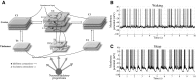
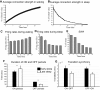
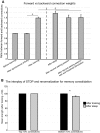
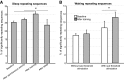
Recent evidence indicates that net synaptic strength in cortical and other networks increases during wakefulness and returns to a baseline level during sleep. These homeostatic changes in synaptic strength are accompanied by corresponding changes in sleep slow wave activity (SWA) and in neuronal firing rates and synchrony. Other evidence indicates that sleep is associated with an initial reactivation of learned firing patterns that decreases over time. Finally, sleep can enhance performance of learned tasks, aid memory consolidation, and desaturate the ability to learn. Using a large-scale model of the corticothalamic system equipped with a spike-timing dependent learning rule, in agreement with experimental results, we demonstrate a net increase in synaptic strength in the waking mode associated with an increase in neuronal firing rates and synchrony. In the sleep mode, net synaptic strength decreases accompanied by a decline in SWA. We show that the interplay of activity and plasticity changes implements a control loop yielding an exponential, self-limiting renormalization of synaptic strength. Moreover, when the model "learns" a sequence of activation during waking, the learned sequence is preferentially reactivated during sleep, and reactivation declines over time. Finally, sleep-dependent synaptic renormalization leads to increased signal-to-noise ratios, increased resistance to interference, and desaturation of learning capabilities. Although the specific mechanisms implemented in the model cannot capture the variety and complexity of biological substrates, and will need modifications in line with future evidence, the present simulations provide a unified, parsimonious account for diverse experimental findings coming from molecular, electrophysiological, and behavioral approaches.
Figures


References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci 3, Suppl: 1178–1183, 2000 - PubMed
-
- Achermann P, Borbély AA. Mathematical models of sleep regulation. Front Biosci 8: s683–693, 2003 - PubMed
-
- Achermann P, Dijk DJ, Brunner DP, Borbély AA. A model of human sleep homeostasis based on EEG slow-wave activity: quantitative comparison of data and simulations. Brain Res Bull 31: 97–113, 1993 - PubMed
-
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol 107: 69–83, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

