Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo
- PMID: 20926669
- PMCID: PMC2988451
- DOI: 10.1523/JNEUROSCI.1144-10.2010
Spatiotemporal coupling between hippocampal acetylcholine release and theta oscillations in vivo
Abstract
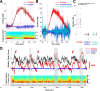
Both acetylcholine (ACh) and theta oscillations are important for learning and memory, but the dynamic interaction between these two processes remains unclear. Recent advances in amperometry techniques have revealed phasic ACh releases in vivo. However, it is unknown whether phasic ACh release co-occurs with theta oscillations. We investigated this issue in the CA1 region of urethane-anesthetized male rats using amperometric and electrophysiological recordings. We found that ACh release was highly correlated with the appearance of both spontaneous and induced theta oscillations. Moreover, the maximal ACh release was observed around or slightly above the pyramidal layer. Interestingly, such release lagged behind theta initiation by 25-60 s. The slow ACh release profile was matched by the slow firing rate increase of a subset of medial-septal low-firing-rate neurons. Together, these results establish, for the first time, the in vivo coupling between phasic ACh release and theta oscillations on spatiotemporal scales much finer than previously known. These findings also suggest that phasic ACh is not required for theta initiation and may instead operate synergistically with theta oscillations to promote neural plasticity in the service of learning and memory.
Figures




Comment in
-
Decoupling of acetylcholine influx and theta power in the hippocampus.J Neurosci. 2011 Mar 9;31(10):3519-21. doi: 10.1523/JNEUROSCI.5862-10.2011. J Neurosci. 2011. PMID: 21389208 Free PMC article. No abstract available.
References
-
- Adams RN. In vivo electrochemical measurements in the CNS. Prog Neurobiol. 1990;35:297–311. - PubMed
-
- Andersen P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. Oxford: Oxford UP; 1996. The hippocampus book.
-
- Apartis E, Poindessous-Jazat FR, Lamour YA, Bassant MH. Loss of rhythmically bursting neurons in rat medial septum following selective lesion of septohippocampal cholinergic system. J Neurophysiol. 1998;79:1633–1642. - PubMed
-
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
