TatB functions as an oligomeric binding site for folded Tat precursor proteins
- PMID: 20926683
- PMCID: PMC2993744
- DOI: 10.1091/mbc.E10-07-0585
TatB functions as an oligomeric binding site for folded Tat precursor proteins
Abstract
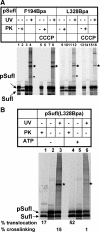
Twin-arginine-containing signal sequences mediate the transmembrane transport of folded proteins. The cognate twin-arginine translocation (Tat) machinery of Escherichia coli consists of the membrane proteins TatA, TatB, and TatC. Whereas Tat signal peptides are recognized by TatB and TatC, little is known about molecular contacts of the mature, folded part of Tat precursor proteins. We have placed a photo-cross-linker into Tat substrates at sites predicted to be either surface-exposed or hidden in the core of the folded proteins. On targeting of these variants to the Tat machinery of membrane vesicles, all surface-exposed sites were found in close proximity to TatB. Correspondingly, incorporation of the cross-linker into TatB revealed multiple precursor-binding sites in the predicted transmembrane and amphipathic helices of TatB. Large adducts indicative of TatB oligomers contacting one precursor molecule were also obtained. Cross-linking of Tat substrates to TatB required an intact twin-arginine signal peptide and disappeared upon transmembrane translocation. Our collective data are consistent with TatB forming an oligomeric binding site that transiently accommodates folded Tat precursors.
Figures




References
-
- Alami M., Lüke I., Deitermann S., Eisner G., Koch H. G., Brunner J., Müller M. Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol. Cell. 2003;12:937–946. - PubMed
-
- Alami M., Trescher D., Wu L. F., Müller M. Separate analysis of twin-arginine translocation (Tat)-specific membrane binding and translocation in Escherichia coli. J. Biol. Chem. 2002;277:20499–20503. - PubMed
-
- Barrett C. M., Freudl R., Robinson C. Twin arginine translocation (Tat)-dependent export in the apparent absence of TatABC or TatA complexes using modified Escherichia coli TatA subunits that substitute for TatB. J. Biol. Chem. 2007;282:36206–36213. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

