Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization
- PMID: 20926790
- PMCID: PMC3077721
- DOI: 10.4049/jimmunol.1001821
Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization
Abstract
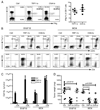
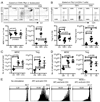
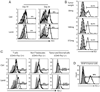
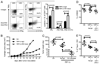
Despite intensive effort, the antitumor efficacy of tumor vaccines remains limited in treating established tumors regardless of the potent systemic tumor-specific immune response and the increases of tumor infiltration of T effector cells. In the current study, we demonstrated that although lentivector (lv) immunization markedly increased Ag-dependent tumor infiltration of CD8 and CD4 T cells and generated Ag-specific antitumor effect, it simultaneously increased the absolute number of myeloid-derived suppressor cells and regulatory T cells in the tumor lesions. In addition, lv immunization induced expression of programmed death-ligand 1 in the tumor lesions. Furthermore, the tumor-infiltrating CD8 T cells expressed high levels of programmed death-1 and were partially dysfunctional, producing lower amounts of effector cytokines and possessing a reduced cytotoxicity. Together, these immune-suppression mechanisms in the tumor microenvironment pose a major obstacle to effective tumor immunotherapy and may explain the limited antitumor efficacy of lv immunization. The loss of effector function in the tumor microenvironment is reversible, and the effector function of CD8 T cells in the tumor could be partially rescued by blocking programmed death-1 and programmed death-ligand 1 pathway in vitro and in vivo, resulting in enhanced antitumor efficacy of lv immunization. These data suggest that immunization alone may exacerbate immune suppression in the tumor lesions and that methods to improve the tumor microenvironment and to rescue the effector functions of tumor-infiltrating T cells should be incorporated into immunization strategies to achieve enhanced antitumor efficacy.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Elsawa SF, Rodeberg DA, Celis E. T-cell epitope peptide vaccines. Expert Rev. Vaccines. 2004;3:563–575. - PubMed
-
- Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. - PubMed
-
- Anderson RJ, Schneider J. Plasmid DNA and viral vector-based vaccines for the treatment of cancer. Vaccine. 2007;25 Suppl 2:B24–B34. - PubMed
-
- Harrop R, John J, Carroll MW. Recombinant viral vectors: cancer vaccines. Adv. Drug Deliv. Rev. 2006;58:931–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

