ERK3 is required for metaphase-anaphase transition in mouse oocyte meiosis
- PMID: 20927325
- PMCID: PMC2947517
- DOI: 10.1371/journal.pone.0013074
ERK3 is required for metaphase-anaphase transition in mouse oocyte meiosis
Abstract
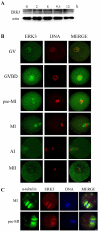
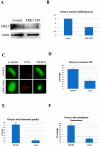
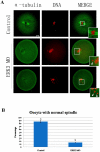
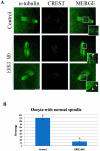
ERK3 (extracellular signal-regulated kinase 3) is an atypical member of the mitogen-activated protein (MAP) kinase family of serine/threonine kinases. Little is known about its function in mitosis, and even less about its roles in mammalian oocyte meiosis. In the present study, we examined the localization, expression and functions of ERK3 during mouse oocyte meiotic maturation. Immunofluorescent analysis showed that ERK3 localized to the spindles from the pre-MI stage to the MII stage. ERK3 co-localized with α-tubulin on the spindle fibers and asters in oocytes after taxol treatment. Deletion of ERK3 by microinjection of ERK3 morpholino (ERK3 MO) resulted in oocyte arrest at the MI stage with severely impaired spindles and misaligned chromosomes. Most importantly, the spindle assembly checkpoint protein BubR1 could be detected on kinetochores even in oocytes cultured for 10 h. Low temperature treatment experiments indicated that ERK3 deletion disrupted kinetochore-microtubule (K-MT) attachments. Chromosome spreading experiments showed that knock-down of ERK3 prevented the segregation of homologous chromosomes. Our data suggest that ERK3 is crucial for spindle stability and required for the metaphase-anaphase transition in mouse oocyte maturation.
Conflict of interest statement
Figures
References
-
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. - PubMed
-
- Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. - PubMed
-
- Sirard MA. Resumption of meiosis: mechanism involved in meiotic progression and its relation with developmental competence. Theriogenology. 2001;55:1241–1254. - PubMed
-
- Doubilet S, McKim KS. Spindle assembly in the oocytes of mouse and Drosophila--similar solutions to a problem. Chromosome Res. 2007;15:681–696. - PubMed
-
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

