The gap gene network
- PMID: 20927566
- PMCID: PMC3016493
- DOI: 10.1007/s00018-010-0536-y
The gap gene network
Abstract
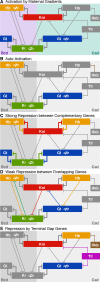
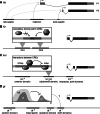
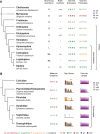
Gap genes are involved in segment determination during the early development of the fruit fly Drosophila melanogaster as well as in other insects. This review attempts to synthesize the current knowledge of the gap gene network through a comprehensive survey of the experimental literature. I focus on genetic and molecular evidence, which provides us with an almost-complete picture of the regulatory interactions responsible for trunk gap gene expression. I discuss the regulatory mechanisms involved, and highlight the remaining ambiguities and gaps in the evidence. This is followed by a brief discussion of molecular regulatory mechanisms for transcriptional regulation, as well as precision and size-regulation provided by the system. Finally, I discuss evidence on the evolution of gap gene expression from species other than Drosophila. My survey concludes that studies of the gap gene system continue to reveal interesting and important new insights into the role of gene regulatory networks in development and evolution.
Figures


References
-
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987;101:1–22. - PubMed
-
- Ingham PW. The molecular genetics of embryonic pattern formation in Drosophila . Nature. 1988;335:25–34. - PubMed
-
- Wolpert L. The French Flag problem: a contribution to the discussion on pattern development and regulation. In: Waddington CH, editor. Towards a theoretical biology. Edinburgh, UK: Edinburgh University Press; 1968. pp. 125–133.
-
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J Theor Biol. 1969;25:1–47. - PubMed
-
- Jaeger J, Reinitz J. On the dynamic nature of positional information. BioEssays. 2006;28:1102–1111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

