Vaginal misoprostol for cervical ripening and induction of labour
- PMID: 20927722
- PMCID: PMC7061246
- DOI: 10.1002/14651858.CD000941.pub2
Vaginal misoprostol for cervical ripening and induction of labour
Abstract
Background: Misoprostol (Cytotec, Searle) is a prostaglandin E1 analogue widely used for off-label indications such as induction of abortion and of labour. This is one of a series of reviews of methods of cervical ripening and labour induction using standardised methodology.
Objectives: To determine the effects of vaginal misoprostol for third trimester cervical ripening or induction of labour.
Search strategy: The Cochrane Pregnancy and Childbirth Group's Trials Register (November 2008) and bibliographies of relevant papers. We updated this search on 30 April 2010 and added the results to the awaiting classification section.
Selection criteria: Clinical trials comparing vaginal misoprostol used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis: We developed a strategy to deal with the large volume and complexity of trial data relating to labour induction. This involved a two-stage method of data extraction.We used fixed-effect Mantel-Haenszel meta-analysis for combining dichotomous data.If we identified substantial heterogeneity (I² greater than 50%), we used a random-effects method.
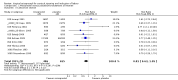
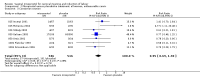
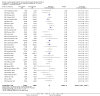
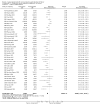
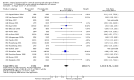
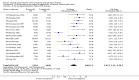
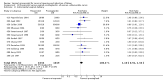
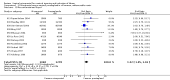
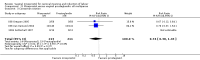
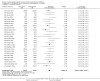
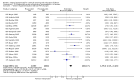
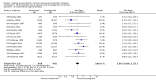
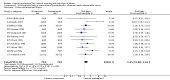
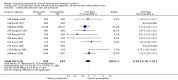
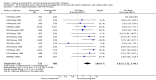
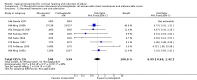
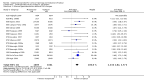
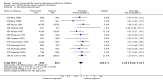
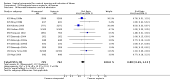
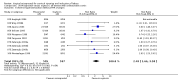
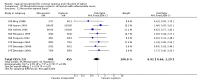
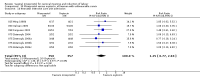
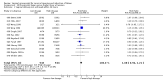
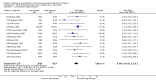
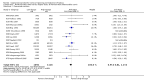
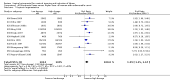
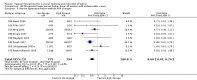
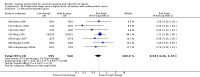
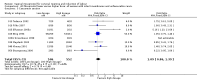
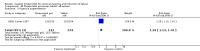
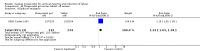
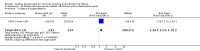
Main results: We included 121 trials. The risk of bias must be kept in mind as only 13 trials were double blind.Compared to placebo, misoprostol was associated with reduced failure to achieve vaginal delivery within 24 hours (average relative risk (RR) 0.51, 95% confidence interval (CI) 0.37 to 0.71). Uterine hyperstimulation, without fetal heart rate (FHR) changes, was increased (RR 3.52 95% CI 1.78 to 6.99).Compared with vaginal prostaglandin E2, intracervical prostaglandin E2 and oxytocin, vaginal misoprostol was associated with less epidural analgesia use, fewer failures to achieve vaginal delivery within 24 hours and more uterine hyperstimulation. Compared with vaginal or intracervical prostaglandin E2, oxytocin augmentation was less common with misoprostol and meconium-stained liquor more common.Lower doses of misoprostol compared to higher doses were associated with more need for oxytocin augmentation and less uterine hyperstimulation, with and without FHR changes.We found no information on women's views.
Authors' conclusions: Vaginal misoprostol in doses above 25 mcg four-hourly was more effective than conventional methods of labour induction, but with more uterine hyperstimulation. Lower doses were similar to conventional methods in effectiveness and risks. The authors request information on cases of uterine rupture known to readers. The vaginal route should not be researched further as another Cochrane review has shown that the oral route of administration is preferable to the vaginal route. Professional and governmental bodies should agree guidelines for the use of misoprostol, based on the best available evidence and local circumstances.
Conflict of interest statement
None known.
Figures


































































































































































































































































































































































































































Update of
-
Vaginal misoprostol for cervical ripening and induction of labour.Cochrane Database Syst Rev. 2003;(1):CD000941. doi: 10.1002/14651858.CD000941. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2010 Oct 06;(10):CD000941. doi: 10.1002/14651858.CD000941.pub2. PMID: 12535398 Updated.
References
References to studies included in this review
006 Ewert 2006 {published data only}
-
- Ewert K, Powers B, Robertson S, Alfirevic Z. Controlled‐release misoprostol vaginal insert in parous woman for labor induction. Obstetrics & Gynecology 2006;108:1130‐7. - PubMed
013 Papanikolaou 2004 {published data only}
013 Tedesco 2002 {published data only}
-
- Tedesco RP, Cecatti JG, Lourenco N, Filho M. Effectiveness of two different doses of vaginal misoprostol for cervical ripening and labor induction [Efetividade de duas diferentes doses de misoprostol por via vaginal para preparo cervical e inducao do parto]. Revista Brasileira de Ginecologia e Obstetricia 2002;24(10):614‐6.
019 Filho 2007 {published data only}
-
- Filho FAR, Alencar Junior CA, Feitosa FE, Arcanjo FCN. Low‐dose vaginal misoprostol (12.5 versus 25mcg) for induction of labor at term [Baixas doses de misoprostol vaginal (12.5 versus 25mcg) para indução de parto a termo]. Revista Brasileira de Ginecologia e Obstetrícia 2007;29(12):639‐46.
025 Elhassan 2005a {published data only}
-
- Elhassan EM, Mirghani OA, Adam I. Cervical ripening and labor induction with 25mcg versus 50mcg of intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2005;90:234‐35. - PubMed
025G Srisomboon 1998 {published data only}
-
- Srisomboon J, Singchai S. A comparison between 25 micrograms and 50 micrograms of intravaginal misoprostol for labor induction. Journal of the Medical Association of Thailand 1998;81(10):779‐82. - PubMed
025 Haghighi 2006 {published data only}
-
- Haghighi L. Intravaginal misoprostol in preterm premature rupture of membranes with low Bishop scores. International Journal of Gynecology & Obstetrics 2006;94(2):121‐2. - PubMed
025 Incerpi 2001 {published data only}
-
- Incerpi M, Fassett M, Kjos S, Tran S, Wing D. Vaginally administered misoprostol for outpatient labor induction in pregnancies with diabetes mellitus [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S120. - PubMed
-
- Incerpi MH, Fassett MJ, Kjos SL, Tran SH, Wing DA. Vaginally administered misoprostol for outpatient cervical ripening in pregnancies complicated by diabetes mellitus. American Journal of Obstetrics and Gynecology 2001;185:916‐9. - PubMed
025 Krupa 2005 {published data only}
-
- Graca Krupa F, Cecatti JG, Castro Surita FG, Milanez HM, Parpinelli MA. Misoprostol versus expectant management in premature rupture of membranes at term. BJOG: an international journal of obstetrics and gynaecology 2005;112(9):1284‐90. - PubMed
025 Kumar 2001 {published data only}
025 Majoko 2001 {published data only}
-
- Majoko F, Zwizwai M, Lindmark G, Nystrom L. A randomised controlled trial of labour induction with misoprostol and prostaglandin F2α gel. 20th Conference on Priorities in Perinatal Care in Southern Africa; 2001 March 6‐9; KwaZulu‐Natal, South Africa. 2001.
025 Majoko 2002c {published data only}
-
- Majoko F, Zwizwai M, Nystrom L, Lindmark G. Vaginal misoprostol for induction of labour: a more effective agent than prostaglandin F2α gel and prostaglandin E2 pessary. Central African Journal of Medicine 2002;48(11‐12):123‐8. - PubMed
025 McKenna 2004 {published data only}
-
- McKenna DS, Ester JB, Poffitt M, Waddell KR. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstetrics & Gynecology 2004;104(3):579‐84. - PubMed
025 Meyer 2005 {published data only}
-
- Meyer M, Pflum J. Outpatient administration of misoprostol decreases induction time. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S167.
-
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstetrics & Gynecology 2005;105:466‐72. - PubMed
025 Oboro 2005 {published data only}
-
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2005;84(7):628‐31. - PubMed
025 Sheela 2007 {published data only}
-
- Sheela CN, Mhaskar A, George S. Comparison of vaginal misoprostol and oral misoprostol with intracervical dinoprostone gel for labour induction at term. Journal of Obstetrics and Gynaecology of India 2007;57(4):327‐30.
025 Stitely 2000 {published data only}
-
- Stitely ML, Browning J, Fowler M, Gendron RT, Gherman RB. Outpatient cervical ripening with intravaginal misoprostol. Obstetrics & Gynecology 2000;96:684‐8. - PubMed
025 Wang 1998 {published data only}
-
- Wang H, Li L, Pu L. The effect of 25mcg misoprostol on induction of labour in late pregnancy. Chinese Journal of Obstetrics and Gynecology 1998;33:469‐71. - PubMed
025 Wing 1996 {published data only}
-
- Wing DA, Paul RH. A comparison of differing dosing regimens of vaginally administered misoprostol for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1996;175:158‐64. - PubMed
025 Wing 1998a {published data only}
-
- Wing DA, Paul RH. Induction of labor with misoprostol for premature rupture of membranes beyond 36 weeks gestation. American Journal of Obstetrics and Gynaecology 1998;179(1):94‐9. - PubMed
-
- Wing DA, Paul RH. Induction of labor with misoprostol for premature rupture of membranes beyond 36 weeks gestation. American Journal of Obstetrics and Gynecology 1998;178:S93. - PubMed
025 Wing 1998b {published and unpublished data}
-
- Wing DA, Lovett K, Paul RH. Disruption of prior uterine incision following misoprostol for labour induction in women with previous cesarean delivery. Obstetrics & Gynecology 1998;91(5 Pt 2):828‐30. - PubMed
030 Moodley 2003 {published data only}
-
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Medical Journal 2003;93:371‐4. - PubMed
038 Aquino 2003 {published data only}
038 Cecatti 2000 {published data only}
-
- Cecatti JG, Aquino MMA, Garcia GM, Rodrigues TMC. Misoprostol versus oxytocin for labor induction: randomized controlled trial. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:28.
038 Clark 1998 {published data only}
-
- Clark A, Cook V, Hill P, Spinnato J. Cervical ripening and labor induction: misoprostol vs dinoprostone. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S30.
038 El‐Sherbiny 2001 {published data only}
-
- El‐Sherbiny M. Vaginal misoprostol for labor induction 25ug versus 50ug dose regimens. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:30.
-
- El‐Sherbiny MT, El‐Gharieb IH, Gewley HA. Vaginal misoprostol for induction of labor: 25 vs. 50 ug dose regimen. International Journal of Gynecology & Obstetrics 2001;72:25‐30. - PubMed
038 Eroglu 2007 {published data only}
-
- Eroglu D, Oktem M, Yanik F, Kuscu E. Labor induction at term: a comparison of the effects of 50 microg and 25 microg vaginal misoprostol. Clinical and Experimental Obstetrics and Gynecology 2007;34(2):102‐5. - PubMed
038 Gregson 2005 {published data only}
-
- Gregson S, Waterstone M, Norman I, Murrells T. A randomised controlled trial comparing low dose vaginal misoprostol and dinoprostone vaginal gel for inducing labour at term. BJOG: an international journal of obstetrics and gynaecology 2005;112:438‐44. - PubMed
038 Has 2002 {published data only}
-
- Has R, Batukan C, Ermis H, Cevher E, Araman A, Kilic G, et al. Comparison of 25 and 50 mcg vaginally administered misoprostol for preinduction of cervical ripening and labor induction. Gynecologic and Obstetric Investigation 2002;53:16‐21. - PubMed
038 Kidanto 2006 {published data only}
-
- Kidanto HL, Kaguta MM, Roosmalen J. Induction of labor with misoprostol or oxytocin in Tanzania. International Journal of Gynecology & Obstetrics 2006;96(1):30‐1. - PubMed
038 Krithika 2008 {published data only}
-
- Krithika KS, Aggarwal N, Suri V. Prospective randomised controlled trial to compare safety and efficacy of intravaginal misoprostol with intracervical cerviprime for induction of labour with unfavourable cervix. Journal of Obstetrics and Gynaecology 2008;28(3):294‐7. - PubMed
038 Meydanli 2003 {published data only}
-
- Meydanli MM, Caliskan E, Burak F, Narin MA, Atmaca R. Labor induction post‐term with 25 micrograms vs 50 micrograms of intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2003;81:249‐55. - PubMed
038 Murthy 2006 {published data only}
-
- Murthy BK, Arkalgud MS. Misoprostol alone versus a combination of dinoprostone and oxytocin for induction of labour. Journal of Obstetrics and Gynaecology of India 2006;56(5):411‐6.
038 Van Gemund 2004 {published data only}
-
- Gemund N, Scherjon S, LeCessie S, Leeuwen JH, Roosmalen J, Kanhai HH. A randomised trial comparing low dose vaginal misoprostol and dinoprostone for labour induction. BJOG: an international journal of obstetrics and gynaecology 2004;111:42‐9. - PubMed
038 Wing 1997 {published data only}
-
- Wing DA, Ortiz‐Omphroy G, Paul RH. A comparison of intermittent vaginal administration of misoprostol with continuous dinoprostone for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;177:612‐8. - PubMed
-
- Wing DA, Paul RH. Vaginally administered misoprostol (Cytotec) versus the dinoprostone vaginal insert (Cervidil) for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;176:S113. - PubMed
043 Diro 1999 {published data only}
-
- Diro M, Adra A, Gilles JM, Nassar A, Rodriguez A, Salamat SM, et al. A double‐blind randomized trial of two dose regimens of misoprostol for cervical ripening and labor induction. Journal of Maternal‐Fetal Medicine 1999;8(3):114‐8. - PubMed
043 Farah 1997 {published data only}
-
- Farah LA, Sanchez‐Ramos L, Rosa C, Valle GO, Gaudier FL, Delke I, et al. Randomised trial of two doses of the prostaglandin E1 analog misoprostol for labor induction. American Journal of Obstetrics and Gynecology 1997;177:364‐9. - PubMed
-
- Sanchez‐Ramos L, Farah L, Rosa C, Johnson J, Delke I, Valle G. Comparative study of a two dose schedule of the PGE1 analogue misoprostol for labor induction in patients with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1996;171(1 Pt 2):319.
044 Chen 2005 {published data only}
-
- Chen DC, Yuan SS, Su HY, Lo SC, Ren SS, Wu GJ. Urinary cyclic guanosine 3',5'‐monophosphate and cyclic adenosine 3',5'‐monophosphate changes in spontaneous and induced onset active labor. Acta Obstetricia et Gynecologica Scandinavica 2005;84:1081‐6. - PubMed
044 Nanda 2007 {published data only}
-
- Nanda S, Singhal SR, Papneja A. Induction of labour with intravaginal misoprostol and prostaglandin E2 gel: a comparative study. Tropical Doctor 2007;37(1):21‐4. - PubMed
044 Wing 1995b {published data only}
-
- Wing DA, Rahall A, Jones MM, Goodwin TM, Paul RH. Misoprostol: an effective agent for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1995;172:1811‐6. - PubMed
048 Khoury 2001 {published data only}
-
- Khoury A, Zhou Q, Gorenberg D, Nies B, Manley G, Mecklenburg F. A randomized clinical trial comparing misoprostol suppositories with continuous dinoprostone for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S118. - PubMed
-
- Khoury AN, Zhou Q‐P, Gorenberg DM, Nies BM, Manley GE, Mecklenburg FE. A comparison of intermittent vaginal administration of two different doses of misoprostol suppositories with continuous dinoprostone for cervical ripening and labor induction. Journal of Maternal Fetal Medicine 2001;10:186‐92. - PubMed
050 Abdul 2007 {published data only}
-
- Abdul MA, Ibrahim UN, Yusuf MD, Musa H. Efficacy and safety of misoprostol in induction of labour in a Nigerian tertiary hospital [L'efficacité et la sûreté de misoprosotl dans l'induction de travail dans un Hôpital Tertiaire Nigerian]. West African Journal of Medicine 2007;26(3):213‐6. - PubMed
050 Agarwal 2003 {published data only}
-
- Agarwal N, Gupta A, Kriplani A, Bhatla N, Parul N. Six hourly vaginal misoprostol versus intracervical dinoprostone for cervical ripening and labor induction. Journal of Obstetrics and Gynaecology Research 2003;29(3):147‐51. - PubMed
050 Ayad 2002 {published data only}
-
- Ayad IAA. Vaginal misoprostol in managing premature rupture of membranes. Eastern Mediterranean Health Journal 2002;8(4 & 5):515‐20. - PubMed
050 Bounyasong 2000 {published data only}
-
- Bounyasong S. A randomized comparison between 25 microgram misoprostol gel and 50 microgram vaginal misoprostol tablet for induction of labour. Thai Journal of Obstetrics and Gynecology 2000;12(1):21‐5.
050 Calder 2008 {published data only}
-
- Calder AA, Loughney AD, Weir CJ, Barber JW. Induction of labour in nulliparous and multiparous women: a UK, multicentre, open‐label study of intravaginal misoprostol in comparison with dinoprostone. BJOG: an international journal of obstetrics and gynaecology 2008;115(10):1279‐88. - PubMed
-
- Calder AA, Loughney AJ, Denison F, Polson D, Erskine K, Dally JJ, et al. A randomised, open‐label comparison of intravaginal (APL202) and dinoprostone for cervical ripening and labour induction in nulliparae. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):58‐9. - PubMed
050 Campos Perez 1994 {published data only}
-
- Campos GA, Guzman S, Rodriguez JG, Voto LS, Margulies M. Misoprostol‐‐a PGE1 analog for induction of labor at term: comparative and randomized study with oxytocin [Misoprostol‐‐un analogo de la PGE1‐‐para la induccion de parto a termino: estudio comparativo y randomizado con oxitocina]. Revista Chilena de Obstetricia y Ginecologia 1994;59:190‐6. - PubMed
-
- Campos Perez GA, Margulies M, Ortega I, Voto LS. Induction of labor with misoprostol, a PGE1 analog. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:97.
-
- Margulies M, Campos Perez G, Voto LS. Misoprostol to induce labour [letter]. Lancet 1992;339:64. - PubMed
050 Charoenkul 2000 {published data only}
-
- Charoenkul S, Sripramote M. A randomized comparison of one single dose of vaginal 50 mcg misoprostol with 3 mg dinoprostone in pre‐induction cervical ripening. Journal of the Medical Association of Thailand 2000;83:1026‐34. - PubMed
050 Denguezli 2007 {published data only}
-
- Denguezli W, Trimech A, Haddad A, Hajjaji A, Saidani Z, Faleh R, et al. Eficacy and safety of six hourly vaginal misoprostol versus intracervical dinoprostone: a randomised controlled trial. Archives of Gynecology and Obstetrics 2007;276(2):119‐24. - PubMed
050 El‐Azeem 1997 {published data only}
-
- El‐Azeem S, Samuels P, Welch G, Staisch K. Term labor induction with PGE1 misoprostol versus PGE2 dinoprostone. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S113.
050 Elhassan 2004 {published data only}
-
- Elhassan M, Mirghani OA, Adam I. Intravaginal misoprostol versus dinoprostone as cervical ripening and labour inducing agents. International Journal of Gynecology & Obstetrics 2004;85:285‐6. - PubMed
050 Elhassan 2005b {published data only}
-
- Elhassan EM, Mirghani OA, Adam I. Misoprostol versus oxytocin for induction of labor. International Journal of Gynecology & Obstetrics 2005;91:254‐5. - PubMed
050 Frohn 2002 {published data only}
-
- Frohn WE, Simmons S, Carlan SJ. Prostaglandin E2 gel versus misoprostol for cervical ripening in patients with premature rupture of membranes after 34 weeks. Obstetrics & Gynecology 2002;99(2):206‐10. - PubMed
050G Carlan 1997 {published data only}
-
- Carlan SJ, Bouldin S, O'Brien WF. Extemporaneous preparation of misoprostol gel for cervical ripening: a randomized trial. Obstetrics & Gynecology 1997;90(6):911‐5. - PubMed
050 Gelisen 2005 {published data only}
-
- Gelisen O, Caliskan E, Dilbaz S, Ozdas E, Dilbaz B, Ozdas E, et al. Induction of labor with three different techniques at 41 weeks of gestation or spontaneous follow up until 42 weeks in women with definitely unfavorable cervical scores. European Journal of Obstetrics & Gynecology and Reprodutive Biology 2005;120(2):164‐9. - PubMed
050 Gotschall 1998 {published data only}
-
- Gottschall D, Borgida AF, Feldman DM, Alberti W, Rodis JF. Preinduction cervical ripening comparing 50 and 100 mcg of misoprostol. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S93. - PubMed
050 Kovavisarach 1997 {published data only}
-
- Kovavisarach E, Wattanasiri S. Comparison of intravaginal misoprostol and dinoprostone for cervical ripening and labour induction at term with unfavorable cervix: a randomized controlled study. Thai Journal of Obstetrics and Gynaecology 1997;9(3):175‐81.
050 Kovavisarach 1998 {published data only}
-
- Kovavisarach E, Worachet W. Randomized controlled trial of intravaginal 50 mcg misoprostol and 3 mg dinoprostone for cervical ripening and labour induction at term with unfavorable cervix. Thai Journal of Obstetrics and Gynaecology 1998;10(1):27‐32.
050 Le Roux 2002 {published data only}
-
- Roux PA, Olarogun JO, Penny J, Anthony J. Oral and vaginal misoprostol compared with dinoprostone for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 2002;99(2):201‐5. - PubMed
050 Lokugamage 2003a {published data only}
-
- Lokugamage AU, Forsyth SF, Sullivan KR, Refaey H, Rodeck CH. Randomized trial in multiparous patients: investigating a singe vs. two‐dose regimen of intravaginal misoprostol for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 2003;82:138‐42. - PubMed
050 Lokugamage 2003b {published data only}
-
- Lokugamage AU, Forsyth SF, Sullivan KR, Refaey H, Rodeck CH. Dinoprostone versus misoprostol: a randomized study of nulliparous women undergoing induction of labour. Acta Obstetricia et Gynecologica Scandinavica 2003;82:133‐7. - PubMed
050 Majoko 2002a {published data only}
-
- Majoko F, Nystrom L, Lindmark G. No benefit, but increased harm from high dose (100ug) misoprostol for induction of labour: a randomised trial of high vs. low (50ug) dose misoprostol. Journal of Obstetrics and Gynaecology 2002;22(6):614‐7. - PubMed
050 Mosquera 1999 {published data only}
-
- Mosquera J, Mesa JC, Navarro H, Cobo E, Neira C, Zuniga J. Study of the efficacy of misoprostol compared with oxytocin for labor induction in women with prolonged amenorrhea [Estudio de la eficacia de misoprostol comparado con oxitocina, en la induccion del parto en la amenorrea prolongada]. Revista Colombiana de Obstetricia y Ginecologia 1999;50(1):7‐12.
050 Neiger 2001 {published data only}
-
- Neiger R, Greaves PC. Comparison between vaginal misoprostol and cervical dinoprostone for cervical ripening and labor induction. Tennessee Medicine 2001;94(1):25‐7. - PubMed
050 Ortiz 2002 {published data only}
-
- Morgan‐Ortiz F, Castro EQ, Martinez CBC, Barraza JB, Ramirez IO. Misoprostol and oxytocin for induction of cervical ripening and labor in patients with term pregnancy and premature membrane rupture [Misoprostol y oxitocina para induccion de madurez cervical y trabajo de parto en pacientes con embarazo a termino y ruptura prematura de membranas]. Ginecologia y Obstetricia de Mexico 2002;70:469‐76. - PubMed
050 Pandis 2001 {published data only}
-
- Pandis GK, Papageorghiou AT, Otigbah CM, Howard RJ, Nikolaides KH. Randomized study of vaginal misoprostol (PGE1) and dinoprostone gel (PGE2) for induction of labour at term. Ultrasound in Obstetrics and Gynaecology 2001;18:629‐35. - PubMed
050 Ramsey 1998 {published data only}
-
- Ramsey PS, Harris DY, Ogburn PL, Heise RH, Magtibay PM, Ramin KD. Comparative cost analysis of prostaglandin analogues dinoprostone and misoprostol as labor preinduction agents [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):182. - PubMed
050 Ramsey 2003 {published data only}
-
- Ramsey P, Harris D, Ogburn P, Heise R, Magtibay P, Ramin K. Comparative efficacy of prostaglandin analogues dinoprostone and misoprostol as labor preinduction agents. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S94.
-
- Ramsey P, Meyer L, Harris D, Ogburn P, Ramin K. Characterization of cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening/labor induction. American Journal of Obstetrics and Gynecology 2001;184(1):S115. - PubMed
-
- Ramsey PS, Harris DY, Ogburn PL, Heise RH, Magtibay PM, Ramin KD. Comparative efficacy and cost of the prostaglandin analogs dinoprostone and misoprostol as labor preinduction agents. American Journal of Obstetrics and Gynecology 2003;188(2):560‐5. - PubMed
050 Ramsey 2005 {published data only}
-
- Ramsey PS, Meyer L, Walkes BA, Harris D, Ogburn Jr PL, Heise RH, et al. Cardiotocographic abnormalities associated with dinoprostone and misoprostol cervical ripening. Obstetrics & Gynecology 2005;105(1):85‐90. - PubMed
050 Rozenberg 2001 {published data only}
-
- Rozenberg P, Chevret S, Goffinet F, Durand‐Zaleski I, Ville Y, Vayssiere C, et al. Induction of labour with a viable infant: a randomised clinical trial comparing intravaginal misoprostol and intravaginal dinoprostone. BJOG: an international journal of obstetrics and gynaecology 2001;108:1255‐62. - PubMed
050 Rozenberg 2004 {published data only}
-
- Rozenberg P, Chevret S, Senat MV, Bretelle F, Bonnal AP, Ville Y. A randomized trial that compared intravaginal misoprostol and dinoprostone vaginal insert in pregnancies at high risks of fetal distress. American Journal of Obsterics and Gynecology 2004;191:247‐53. - PubMed
050 Sahu 2004 {published data only}
-
- Sahu L, Chakravertty B. Comparison of prostaglandin E1 (misoprostol) with prostaglandin E2 (dinoprostone) for labour induction. Journal of Obsterics and Gynecology of India 2004;54(2):139‐42.
050 Sifakis 2007 {published data only}
-
- Sifakis S, Angelakis E, Avgoustinaks E, Fragouli Y, Mantas N, Koukoura O, et al. A randomized comparison between intravaginal misoprostol and prostaglandin E2 for labour induction. Archives of Gynecology and Obstetrics 2007;275:263‐7. - PubMed
050 Surbek 1997 {published data only}
-
- Surbek DV, Boesiger H, Hoesli I, Pavic N, Holzgreve W. A double‐blind comparison of the safety and efficacy of intravaginal misoprostol and prostaglandin E2 to induce labor. American Journal of Obstetrics and Gynecology 1997;177(5):1018‐23. - PubMed
-
- Surbek DV, Bosiger H, Hosli I, Pavic N, Holzgreve W. Cervical priming and labor induction with intravaginal misoprostol versus PGE2: a double‐blind randomized trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S112. - PubMed
-
- Surbek DV, Bosiger H, Pavic N, Hosli I, Stoz F, Holzgreve W. The safety of misoprostol for labor induction. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167):36.
-
- Surbek DV, Bosiger H, Pavic N, Stoz F, Holzgreve W. Misoprostol (Cytotec) for labor induction in term pregnancies [Misoprostol (Cytotec) zur geburtseinleitung am termin]. 20th Congress of the Swiss Society of Gynecology and Obstetrics; 1997 June; Lugano, Switzerland. 1997:11.
050 Thomas 2000 {published data only}
-
- Thomas N, Longo SA, Rumney PJ, Nageotte MP, Asrat T. Intravaginal misoprostol in prelabor rupture of membranes at term. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S136.
058 Ferguson 2002 {published data only}
-
- Ferguson J, Head B, Frank F, Frank M, Singer J, Stefos T, et al. Misoprostol versus low‐dose oxytocin for cervical ripening: a prospective, randomized, double‐masked trial. American Journal of Obstetrics and Gynecology 2002;187:273‐80. - PubMed
063 Varaklis 1995 {published data only}
-
- Varaklis K, Gumina R, Stubblefield PG. Randomized controlled trial of vaginal misoprostol and intracervical prostaglandin E2 gel for induction of labor at term. Obstetrics & Gynecology 1995;86:541‐4. - PubMed
075 Buser 1997 {published data only}
-
- Arias F, Buser D, Mora G. Randomized comparison of misoprostol vs dinoprostone for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S141. - PubMed
-
- Buser D, Mora G, Arias F. A randomized comparison between misoprostol and dinoprostone for cervical ripening and labor induction in patients with unfavorable cervices. Obstetrics & Gynecology 1997;89(4):581‐5. - PubMed
075 Chang 1997 {published data only}
-
- Chang CH, Chang FM. Randomized comparison of misoprostol and dinoprostone for preinduction cervical ripening and labor induction. Journal of the Formosan Medical Association 1997;96:366‐9. - PubMed
075 Chuck 1995 {published data only}
-
- Chuck F, Huffaker J. Labor induction with intravaginal prostaglandin E1 (PGE1) (Misoprostol, Cytotec) vs intracervical prostaglandin E2 (PGE2) (Dinoprostone, Prepidil gel): a randomized comparison. American Journal of Obstetrics and Gynecology 1995;172:424. - PubMed
-
- Chuck FJ, Huffaker BJ. Labor induction with intravaginal misoprostol versus intracervical prostaglandin E2 gel (Prepidil gel): randomized comparison. American Journal of Obstetrics and Gynecology 1995;173:1137‐42. - PubMed
075 Danielian 1999 {published data only}
-
- Danielian P, Porter B, Ferri N, Summers J, Templeton A. Misoprostol for induction of labour at term: a more effective agent than dinoprostone vaginal gel. British Journal of Obstetrics and Gynaecology 1999;106(8):793‐7. - PubMed
-
- Danielian PJ, Porter B. Induction of labour with misoprostol. Journal of Obstetrics and Gynaecology 1998;18(Suppl 1):S18‐S19.
075 Escudero 1997 {published data only}
-
- Escudero F, Contreras H. A comparative trial of labor induction with misoprostol versus oxytocin. International Journal of Gynecology & Obstetrics 1997;57:139‐43. - PubMed
075 Fuchs 2006 {published data only}
-
- Fuchs K, Brard L, Hodgman D, Silver H. Prostaglandin E1 gel vs. oxytocin for induction of labor at term. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S101.
075 Ghidini 2001 {published data only}
-
- Ghidini A, Spong CY, Korker V, Mariani E. Randomized controlled trial of 50 and 100 mcg of misoprostol for induction of labor at term. Archives of Gynecology and Obstetrics 2001;265:128‐30. - PubMed
075 Kolderup 1999 {published data only}
-
- Kolderup L, McLean L, Grullon K, Safford K, Kilpatrick SJ. Misoprostol is more efficacious for labor induction than prostaglandin E2, but is it associated with more risk?. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 1):1543‐50. - PubMed
075 Lemancewicz 1999 {published data only}
-
- Lemancewicz A, Urban R, Scotnicki MZ, Karpiuk A, Urban J. Uterine and fetal Doppler flow changes after misoprostol and oxytocin therapy for induction of labor in post‐term pregnancies. International Journal of Gynecology & Obstetrics 1999;67:139‐45. - PubMed
075 Magtibay 1998 {published data only}
-
- Magtibay P, Ogburn P, Harris D, Suman V, Ramin K. Misoprostol as a labor induction agent: a pilot study comparing efficacy, safety and cost. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):327.
-
- Magtibay PM, Ramin KD, Harris DY, Ramsey PS, Ogburn PL. Misoprostol as a labor induction agent. Journal of Maternal Fetal Medicine 1998;7(1):15‐8. - PubMed
075 Megalo 2004 {published data only}
-
- Megalo A, Petignat P, Hohlfeld P. Influence of misoprostol or prostaglandin E2 for induction of labor on the incidence of pathological CTG tracing: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;116:34‐8. - PubMed
075 Mundle 1996 {published data only}
-
- Mundle WR, Young DC. Vaginal misoprostol for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 1996;88(4 Pt 1):521‐5. - PubMed
075 Reyna‐Villasmil 2005 {published data only}
-
- Reyna‐Villasmil E, Guerra‐Velasquez M, Torres‐Montilla M, Reyna‐Villasmil N, Mejia‐Montilla J, Labarca‐Vincero N. Comparative study of intravaginal misoprostol effect at 50 and 100 mcg in cervical ripening and labour induction [Estudio comparativo del efecto del misoprostol intravaginal a dosis de 50 y 100 mcg en la maturación cervical y la inducción del parto]. Investigacíon Clínica 2005;46(2):179‐86. - PubMed
075 Sahin 2002 {published data only}
-
- Sahin HG, Sahin HA, Kocer M. Induction of labor in toxemia with misoprostol. Acta Obstetricia et Gynecologica Scandinavica 2002;81:252‐7. - PubMed
075 Saleen 2006 {published data only}
-
- Saleem S. Efficacy of dinoprostone, intracervical foleys and misoprostol in labour induction. Journal of the College of Physicians and Surgeons Pakistan 2006;16(4):276‐9. - PubMed
075 S‐Ramos 1997 {published data only}
-
- Sanchez‐Ramos L, Chen A, Briones D, Valle GO, Gaudier FL, Delke I. Premature rupture of membranes at term: induction of labor with intravaginal misoprostol tablets (PGE1) or intravenous oxytocin. American Journal of Obstetrics and Gynecology 1994;170:377.
-
- Sanchez‐Ramos L, Chen AH, Kaunitz AM, Gaudier FL, Delke I. Labor induction with intravaginal misoprostol in term premature rupture of membranes: a randomized study. Obstetrics & Gynecology 1997;89:909‐12. - PubMed
075 Tabor 1995 {published data only}
-
- Tabor B, Anderson J, Stettler B, Wetwiska N, Howard T. Misoprostol vs prostaglandin E2 gel for cervical ripening. American Journal of Obstetrics and Gynecology 1995;172:425.
075 Urban 2003 {published data only}
-
- Urban R, Lemancewicz A, Urban J, Skotnicki MZ, Kretowska M. Misoprostol and dinoprostone therapy for labor induction: a Doppler comparison of uterine and fetal hemodynamic effects. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:20‐4. - PubMed
075 Webb 1997 {published data only}
-
- Webb GW, Raynor BD, Huddleston JF, Fandall HW. Induction of labor with an unfavorable cervix: a randomized prospective trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S22.
075 Zeteroglu 2004 {published data only}
-
- Zeteroglu S, Sahin HG, Sahin HA. Induction of labour with misoprostol in grand multiparous patients. International Journal of Gynecology & Obstetrics 2004;87:155‐6. - PubMed
075 Zeteroglu 2006a {published data only}
-
- Zeteroglu S, Engin‐Üstün Y, Üstün Y, Güvercinçi M, Sahin G, Kamaci M. A prospective randomized study comparing misoprostol and oxytocin for premature rupture of membranes at term. Journal of Maternal‐Fetal and Neonatal Medicine 2006;19(5):283‐7. - PubMed
075 Zeteroglu 2006b {published data only}
-
- Zeteroglu S, Sahin HG, Sahin HA. Induction of labour in great multipara with misoprostol. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;126(1):27‐32. - PubMed
075 Zeteroglu 2006c {published data only}
-
- Zeteroglu S, Sahin GH, Sahin HA. Induction of labor with misoprostol in pregnancies with advanced maternal age.. European Journal of Obstetrics & Gynecology and Reproductive Health 2006;129(2):140‐4. - PubMed
088 Garry 2003 {published data only}
-
- Garry D, Figueroa R, Kalish RB, Catalano CJ, Maulik D. Randomized controlled trial of vaginal misoprostol versus dinoprostone vaginal insert for labor induction. Journal of Maternal‐Fetal and Neonatal Medicine 2003;13:254‐9. - PubMed
088 Pi 1999 {published data only}
-
- Pi P, Zhu F. Clinical observation of misoprostol on induction in late pregnancy. Bulletin of Humam Medical University 1999;24:195‐7. - PubMed
088 Saggaf 2001 {published data only}
-
- Saggaf A, Rouzi AA, Radhan B, Alshehry S, Yamani T, Abduljabbar H. Misoprostol for preinduction cervical ripening and induction of labor: a randomized controlled trial. Saudi Journal of Obstetrics and Gynecology 2001;1(2):89‐93.
088 Sanchez Ramos 1998 {published data only}
-
- Sanchez‐Ramos L, Peterson DE, Delke I, Gaudier FL, Kaunitz AM. Labor induction with prostaglandin E1 misoprostol compared with dinoprostone vaginal insert: a randomized trial. Obstetrics & Gynecology 1998;91(3):401‐5. - PubMed
088 Wing 1995a {published data only}
-
- Wing DA, Jones MM, Rahall A, Goodwin TM, Paul RH. A comparison of misoprostol and prostaglandin E2 gel for preinduction cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1995;172:1804‐10. - PubMed
100 Fletcher 1994 {published data only}
-
- Fletcher H, Mitchell S, Frederick J, Simeon D, Brown D. Intravaginal misoprostol versus dinoprostone as cervical ripening and labor‐inducing agents. Obstetrics & Gynecology 1994;83:244‐7. - PubMed
100G Fletcher 1993 {published data only}
-
- Fletcher HM, Mitchell S, Simeon D, Frederick J, Brown D. Intravaginal misoprostol as a cervical ripening agent. British Journal of Obstetrics and Gynaecology 1993;100:641‐4. - PubMed
100G Gottschall 1997 {published data only}
-
- Gottschall D, Borgida AF, Mihalek JJ, Sauer F, Rodis JF. Misoprostol versus prostin E2 gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1997;176:S141. - PubMed
-
- Gottschall DS, Borgida AF, Mihalek JJ, Sauer F, Rodis JF. A randomized clinical trial comparing misoprostol with prostaglandin E2 gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1997;177(5):1067‐70. - PubMed
100G Srisomboon 1996 {published data only}
-
- Srisomboon J, Tongsong T, Tosiri V. Preinduction cervical ripening with intravaginal prostaglandin E1 analogue misoprostol: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 1996;22(2):119‐24. - PubMed
100G Srisomboon 1997 {published data only}
-
- Srisomboon J, Piyamongkol W, Aiewsakul P. Comparison of intracervical and intravaginal misoprostol for cervical ripening and labour induction in patients with an unfavourable cervix. Journal of the Medical Association of Thailand 1997;80(3):189‐94. - PubMed
100 Herabutya 1997 {published data only}
-
- Herabutya Y, O‐Prasertsawat P, Pokirom J. A comparison of intravaginal misoprostol and intracervical prostaglandin E2 gel for ripening of unfavourable cervix and labor induction. Journal of Obstetrics and Gynaecology Researc 1997;23:369‐74. - PubMed
100 Howarth 1996 {published data only}
-
- Howarth GR, Funk M, Steytler P, Pistorius L, Makin J, Pattinson RC. A randomised controlled trial comparing vaginally administered misoprostol to vaginal dinoprostone gel in labour induction. Journal of Obstetrics and Gynaecology 1996;16:474‐8.
-
- Steytler P, Howarth G, Makin J. Cervical ripening and labour induction. Randomised controlled trial comparing misoprostol and dinoprostone vaginal gel. Proceedings of the 14th Conference on Priorities in Perinatal Care in South Africa; 1995 March 7‐10; South Africa. 1995:167‐70.
-
- Steytler P, Howarth GR, Funk M, Pistorius L, Makin J, Pattinson RC. A randomised control trial comparing vaginally administered misoprostol to vaginal dinoprostone gel in labour induction. 15th Conference on Priorities in Perinatal Care in Southern Africa; 1996 March 5‐8; Goudini Spa, South Africa. 1996:13‐4.
100 Montealegre 1999 {published data only}
-
- Montealegre JA, Botero LF, Sabogal G. Labor induction with unfavorable cervix: randomized controlled trial double blind method. Oxitocyn vs. misoprosto [Induccion de trabajo de parto con cervix desfavorable experimento clinico aleatorizado doble ciego de oxiotocina vs microprostol]. Revista Colombiana de Obstetricia y Ginecologia 1999;50(3):133‐7.
100 Ozsoy 2004 {published data only}
-
- Ozsoy M, Ozsoy D. Induction of labor with 50 and 100mcg of misoprostol: comparison of maternal and fetal outcomes. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;113:41‐4. - PubMed
138 Nunes 1999 {published data only}
-
- Nunes F, Rodrigues R, Meirinho M. Randomised comparison between intravaginal misoprostol and dinoprostone for cervical ripening and induction of labour. American Journal of Obstetrics and Gynecology 1999;181(3):626‐9. - PubMed
150 De la Torre 2001 {published data only}
-
- Torre S, Gilson GJ, Flores S, Curet LB, Qualls CE, Rayburn WF. Is high‐dose misoprostol able to lower the incidence of caesarean section? A randomized controlled trial. Journal of Maternal Fetal Medicine 2001;10(2):85‐90. - PubMed
-
- Kramer RL, Gilson G, Morrison DS, Martin D, Gonzales JL, Curet LB. A randomized trial of misoprostol and oxytocin for induction of labor: safety and efficacy. Obstetrics & Gynecology 1997;176(1 Pt 2):S111. - PubMed
-
- Kramer RL, Gilson GJ, Morrison DS, Martin D, Gonzales JL, Qualls CR. A randomized trial of misoprostol and oxytocin for induction of labor: safety and efficacy. Obstetrics & Gynecology 1997;89(3):387‐91. - PubMed
150 Kulshreshtha 2007 {published data only}
-
- Kulshreshtha S, Sharma P, Mohan G, Singh S, Singh S. Comparative study of misoprostol versus dinoprostone for induction of labour. Indian Journal of Physiology and Pharmacology 2007;51(1):55‐61. - PubMed
150 Ngai 2000 {published data only}
-
- Ngai SW, Chan YM, Lam SW, Loa TT. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of membranes. BJOG: an international journal of obstetrics and gynaecology 2000;107(2):222‐7. - PubMed
175 Kadanali 1996 {published data only}
-
- Kadanali S, Kucukozkan T, Zor N, Kumtepe Y. Comparison of labor induction with misoprostol vs. oxytocin/prostaglandin E2 in term pregnancy. International Journal of Gynecology & Obstetrics 1996;55:99‐104. - PubMed
200 Lee 1997 {published data only}
-
- Lee HY. A randomised double‐blind study of vaginal misoprostol vs dinoprostone for cervical ripening and labour induction in prolonged pregnancy. Singapore Medical Journal 1997;38(7):292‐4. - PubMed
200 Rowlands 2001 {published data only}
-
- Rowlands S, Bell R, Donath S, Morrow S, Trudinger BJ. Misoprostol versus dinoprostone for cervical priming prior to induction of labour in term pregnancy: a randomised control trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2001;41(2):145‐52. - PubMed
References to studies excluded from this review
Adair 1998 {published data only}
-
- Adair CD, Weeks JW, Barrilleaux PS, Philibert L, Edwards MS, Lewis DF. Labor induction with oral versus vaginal misoprostol: a randomized, double‐blind trial. American Journal of Obstetrics and Gynecology 1998;178:S93. - PubMed
Aggarwal 2006 {published data only}
-
- Aggarwal N, Kirthika KS, Suri V, Malhotra S. Comparative evaluation of vaginal PGE‐1 analogue (misoprostol) and intracervical PGE‐2 gel for cervical ripening and induction of labor. 49th All India Congress of Obstetrics and Gynaecology; 2006 Jan 6‐9; Cochin, Kerala State, India. 2006:95.
Arrieta 2000 {published data only}
-
- Arrieta OB, Yances BR, Ciodaro CM, Penaranda WA, Aguilera JB. Induction of labor at term with misoprostol vs oxytocin [Induccion de trabajo de parto con microprostol vs. oxitocina]. Revista Colombiana de Obstetricia y Ginecologia 2000;51(1):8‐11.
Azeem 2006 {published data only}
-
- Azeem S. Buccal vs intravaginal misoprostol administration for cervical ripening in induction of labor. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:95.
Balintona 2001 {published data only}
-
- Ballintona J, Meyer L, Ramin K, Vasdev G, Ramsey P. Cardiotocographic abnormalities associated with labor induction. Anesthesiology 2001; Vol. 94, issue 1A:A67.
Belfrage 2000 {published data only}
-
- Belfrage P, Smedvig E, Gjessing L, Eggebo T, Okland I. A randomized prospective study of misoprostol and dinoproston for induction of labour. Acta Obstetricia et Gynecologica Scandinavica 2000;79:1065‐8. - PubMed
Bi 2000 {published data only}
-
- Bi SH, Xu KH, Xing AY, Liu Y. Labour induced by low dose misoprostol in late gestation: a randomized controlled trial. Journal of West China University of Medical Science 2000;31(4):518‐9.
Bolnick 2002a {published data only}
-
- Bolnick J, Velazquez M, Gonzalez J, Leslie K, Rappoport V, McIlwane G, et al. Randomized trial of sustained‐release vaginal dinoprostone (pge2) with concurrent oxytocin versus vaginal misoprostol (pg1) for induction of labor at term. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S175.
Bugalho 1995 {published data only}
-
- Bugalho A, Bique C, Machungo F, Bergstrom S. Vaginal misoprostol as an alternative to oxytocin for induction of labor in women with late fetal death. Acta Obstetricia et Gynecologica Scandinavica 1995;74:194‐8. - PubMed
Butler 2004 {published data only}
-
- Butler B, Crane J, Delaney T. Induction of labour with misoprostol in women at term with an unfavorable cervix: a randomized comparison of oral and vaginal administration [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S190.
Cecatti 2001 {published data only}
-
- Cecatti JG, Faundes A, Pires HMB, Calderon IMP. Labor induction in women with unripe cervix using two products containing misoprostol. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):283.
Cecatti 2006 {published data only}
-
- Cecatti JG, Tedesco RP, Pires HMB, Calderon IM, Faúndes A. Effectiveness and safety of a new vaginal misoprostol product specifically labeled for cervical ripening and labor induction. Acta Obstetricia et Gynecologica 2006;85:706‐11. - PubMed
Cetin 1997 {published data only}
-
- Cetin A, Cetin M, Taskurt A, Izgik E. Misoprostol versus dinoprostone for labor induction in term pregnancies [Term gebeliklerde dogum induksiyonunda dinoproston yerine misoprostol kullanimi]. Jinekoloji ve Obstetrik Dergisi 1997;11:51‐4.
Chang 2003 {published data only}
-
- Chang YK, Chen WH, Yu MH, Liu HS. Intracervical misoprostol and prostaglandin E2 for labor induction. International Journal of Gynecology & Obstetrics 2003;80:23‐8. - PubMed
Chen 2000 {published data only}
-
- Chen LY. Comparison between the oral and vaginal administration of misoprostol for labour induction at term. Journal of Shanghai Medical University 2000;27(1):77‐8.
Chen 2001 {published data only}
-
- Chen P, Zhai GR, Liu M. Clinical study of labour induced by low dose of misoprostol. Journal of Henan Medical College for Staff and Workers 2001;13(2):132.
Chen 2003 {published data only}
-
- Chen JT. A comparison between two dose of vaginal administration of misoprostol for labor induction at term. Xian Dai Shi Yong Yi Xue 2003;15(10):639.
Chen 2004 {published data only}
-
- Chen DC, Ku CH, Chen CH, Wu GJ. Urinary nitric oxide metabolite changes in spontaneous and induced onset active labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:641‐6. - PubMed
Cui 2001 {published data only}
-
- Cui XJ. Possibility and safety of application of misoprostol in the induction of pregnant women with pre‐eclampsia. Progress in Obstetetric and Gynecology 2001;10(2):126‐8.
Dai 2005 {published data only}
-
- Dai JR. Analysis of three methods for induction of labor in 330 cases. Chinese Journal of Maternity and Child Health 2005;20(24):3029.
Delaney 2001 {published data only}
-
- Delaney T, Crane J, Hutchens D, Fanning C, Young D. Induction of labor with intravaginal misoprostol: a comparison of dosing intervals. American Journal of Obstetrics and Gynecology 2001; Vol. 185, issue 6 Suppl:S202.
Ding 2001 {published data only}
-
- Ding HF, Xang XJ. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Wan Nan Medical College 2001;20(1):31‐2.
Ding 2005 {published data only}
-
- Ding DC, Hsu S, Su HY. Low dose intravaginal misoprostol for induction of labor at term. International Journal of Gynecology and Obstetrics 2005;90(1):71‐3. - PubMed
Ding 2006 {published data only}
-
- Ding YH, Zhan HQ. Clinical observation on three induced labour methods for late pregnancy. Clinical Medicine 2006;26(6):9‐10.
Du 2000 {published data only}
-
- Du M. Clinical study of labour induced by low dose misoprostol in late gestation. Chinese Journal Coal Industry Medicine 2000;3(4):301‐2.
Dundas 2000 {published data only}
-
- Dundas K, Howe D, Hughes R. Misoprostol for induction of labour in primigravidae [abstract]. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S50.
Duru 1997 {published data only}
-
- Duru N, Atay V, Pabuccu R, Ergun A, Tokac G, Aydin BA. Vaginal misoprostol versus oxytocin‐prostaglandin e2 gel in severe preeclampsia remote from term. Acta Obstetricia et Gynecologica Scandinavica 1997;76(176):37.
Echeverria 1995 {published data only}
-
- Echeverria E, Rocha M. Randomised comparative study of induced labor with oxytocin and misoprostol in prolonged pregnancies. Revista Chilena de Obstetricia y Ginecologia 1995;60:108‐11. - PubMed
Eftekhavi 2002 {published data only}
-
- Eftekhavi N. A comparison of vaginal misoprostol with intravenous oxytocin for cervical ripening and labor induction [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):47‐8.
El‐Din 2000 {published data only}
-
- El‐Din NMN, Moghazt DAM. Cervical ripening and induction of labour with misoprostol, prostaglandin E2 or prostaglandin E2 gel: a randomized comparative clinical trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:29.
Escalante 1993 {published data only}
-
- Escalante G, Ribas D, Esquivel A, Moya R, Sanchez LO, Pena YC. Misoprostol intracervical or vaginal. Clinical characteristics in delivery induction [Misoprostol intracervical vs. vaginal: caracteristicas clinicas en la induccion del parto]. Revista Costarricense de Ciencias Medicas 1993;14(3):43‐50.
Fonseca 2007 {published data only}
-
- Fonseca L, Lucas M, Wood H, Phat'Ak D, Susan R, Gilstrap L, et al. RCT of misoprostol pre‐induction ripening vs oxytocin induction. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S106, Abstract no: 348.
-
- Fonseca L, Wood HC, Lucas MJ, Ramin SM, Patak D, Gilstrap III LC, et al. Randomized trial of preinduction cervical ripening: misoprostol vs oxytocin. American Journal of Obstetrics and Gynecology 2008;199:305‐e1‐305‐e5. - PubMed
Girija 2006 {published data only}
-
- Girija S, Manjunath AP. Randomized controlled trial of vaginal misoprostol: single 50 mcg dose versus multiple 25 mcg dose for labour induction. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:40.
Gorzelac 1999 {published data only}
-
- Leszczynska‐Gorzelak B, Laskowska M, Oleszczuk J. Using of misoprostol for preinduction and induction in term pregnancy. Ginekologia Polska 1999;70(12):881‐9. - PubMed
Gorzelac 2001 {published data only}
-
- Leszczynska‐Gorzelak B, Laskowska M, Oleszczuk J. Comparative analysis of the effectiveness of misoprostol and prostaglandin E2 in the pre‐induction and induction of labor. Medical Science Monitor 2001;7(5):1023‐8. - PubMed
Harms 2001 {published data only}
-
- Harms K, Nguyen C, Toy EC, Baker B. Intravaginal misoprostol versus cervidil for cervical ripening in term pregnancies [abstract]. Obstetrics & Gynecology 2001; Vol. 97, issue 4 Suppl:36S.
Hoesli 2003 {published data only}
-
- Hoesli I, Gairing A, Lapaire O, Tercanli S, Holzgreve W. Induction of labour: cervical length measurement beside misoprostol or dinoproston ‐ is it a reliable factor both for patients and their obstetrical team?. Ultrasound in Obstetrics & Gynecology 2003;22(Suppl 1):149.
How 2001 {published data only}
-
- How H, Leaseburge L, Khoury J, Siddiqi T, Sibai B. Is there an ideal route of misoprostol administration for cervical ripening and labor indoction?. American Journal of Obstetrics and Gynecology 2001;184(1):S118. - PubMed
Hu 2005 {published data only}
-
- Hu JL, Liu ML, Zhang MX. Study on the medication of misoprostol to induce premature rupture of membranes at term. Practical Clinical Medicine 2005;6(8):71‐4.
Jackson 1999 {published data only}
-
- Jackson N, Irvine R, Edmonds D, Paterson‐Brown S. Random allocation controlled trial of intravaginal misoprostol versus intravaginal dinoprostone for the induction of labour. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S52.
-
- Jackson NV, Irvine R, Paterson‐Brown S. Randomised, controlled trial of intravaginal misoprostol versus intravaginal dinoprostone gel in the induction of labour at term. Womens Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 Oct 3‐6; Cape Town, South Africa. 1999:32.
-
- Jackson NV, Terzidou V, Irvine RV, Edmonds DK, Paterson‐Brown S. Randomised controlled trial of intravaginal misoprostol versus intravaginal dinoprostone for the induction of labour. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 4). 2000:29‐30.
Jazayeri 2003 {published data only}
-
- Jazayeri A, Jazayeri M, Jamal A, Eslamian L, Maoorsi V, Borna S. Prospective randomized clinical trial of cervical ripening with misoprostol for either 8 or 24 hours. American Journal of Obstetrics and Gynecology 2003;6 Suppl 1:S70.
Jouatte 2000 {published data only}
-
- Jouatte F, Subtil D, Marquis P, Plennevaux JL, Puech F. Medical indications of labor induction: a comparison between intravaginal misoprostol and intravenous dinoprostone [Declenchement du travail d'indication medicale: comparaison du misoprostol intravaginal avec une prostaglandine E2 administree par voie intraveineuse]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 2000;29:763‐71. - PubMed
Kwon 1999 {published data only}
-
- Kwon JS, MacKenzie VP, Davies GAL. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomized trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S128. - PubMed
Li 2003 {published data only}
-
- Li SJ. Clinical study of different dose of misoprostol for induction of labour. China Clinical Medicine Research Journal 2003;94:9375‐6.
Liu 1998 {published data only}
-
- Liu D, Wang X. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Changzhi Medical College 1998;12(4)(4):282‐4.
Liu 2004 {published data only}
-
- Liu YJ. Clinical study on the induction of labour by low dose misoprostol in 100 cases. Chinese Journal of Today's Medicine 2004;4(3):33‐4.
Li XQ 2003 {published data only}
-
- Li XQ 2003. Clinical study of labour induced by low dose misoprostol in late gestation. China Clinical Medicine Research Journal 2003;96:9632‐3.
Lulu 1999 {published data only}
-
- Lulu D, Zaiju H. Observation on the efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy and nursing care. Journal of Nursing Science 1999;14:1‐4.
Luo 2000 {published data only}
-
- Luo LM, Wu QK, Tao MF, Shen GF. Study on increasing safety of misoprostol for induction of labour in the third trimester of pregnancy. Shanghai Sheng Wu Yi Xue Gong Cheng Zha Zhi 2000;21(1):50‐3.
Majoko 2002b {published data only}
-
- Majoko F, Zwizwai M, Lindmark G, Nystrom L. Labor induction with vaginal misoprostol and extra‐amniotic prostaglandin F2 alpha gel. International Journal of Gynecology & Obstetrics 2002;76:127‐33. - PubMed
Megalo 1998 {published data only}
-
- Megalo A, Hohlfeld P. Misoprostol (PGE1) as an alternative to PGE2 for pre‐induction cervical ripening and labour induction. 21st Conference of the Swiss Society of Gynecology and Obstetrics. 1998:19.
Megalo 1999 {published data only}
-
- Megalo A, Hohlfield P. Cervical ripening and induction of labour with misoprostol (PG1): effects on the cardiotocogram [Maturation du col et induction du travail par le misoprostol (PGE1): effets sur le cardiotocogramme]. Gynakologisch‐Geburtshilfliche Rundschau 1999;39:165.
Molina 2000 {published data only}
-
- Molina M, Perez R, Fraenkel K, Vergara X. Oxitocin and misoprostol in the inducement of the delivery work of full‐term pregnant women. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:95.
Ngai 1996 {published data only}
-
- Ngai SW, To WK, Lao T, Ho PC. Cervical priming with oral misoprostol in pre‐labor rupture of membranes at term. Obstetrics & Gynecology 1996;87:923‐6. - PubMed
Nuthalapaty 2005 {published data only}
Ozgur 1997 {published data only}
-
- Ozgur K, Kizilates A, Uner M, Erman O, Trak B. Induction of labor with intravaginal misoprostol versus intracervical dinoprostone. Archives of Gynecology and Obstetrics 1997;261:9‐13. - PubMed
Patel 2000 {published data only}
-
- Patel A, Gilles JM, Moffett D, Mahram R, Diro M, Burkett G. Can misoprostol be interchanged with oxytocin for augmentation of labor?. Obstetrics & Gynecology 2000;95(4 Suppl):10S.
Perry 1998 {published data only}
-
- Perry KG, Larmon JE, May WL, Robinette LG, Martin RW. Cervical ripening: a randomized comparison between intravaginal misoprostol and intracervical balloon catheter combined with intravaginal dinoprostone. American Journal of Obstetrics and Gynecology 1998;178:1333‐40. - PubMed
Perry 1999 {published data only}
-
- Perry KG, Larmon JE, Rinehart BK, Gebhart LD, May WL, Martin RW. Cervical ripening: a randomized clinical trial of an intracervical balloon catheter combined with either intravaginal dinoprostone or misoprostol. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. - PubMed
Porojanova 2005 {published data only}
-
- Porojanova V, Sampath J, Porojanova K. Misoprostol and induction of labour. Akusherstvo i Ginekologiia 2005;44(5):27‐30. - PubMed
Roy 2003 {published data only}
-
- Roy G, Ferreira E, Hudon L, Marquette G. The efficacy of oral versus vaginal misoprostol for second‐trimester termination of pregnancy: a double‐blind, randomized placebo‐controlled trial. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S70.
Rust 1999 {published data only}
-
- Rust OA, Greybush M, Singleton C, Atlas RO, Baldicci J. A comparison of preinduction cervical ripening techniques. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S126.
Rust 2000 {published data only}
-
- Rust O, Greybush M, Atlas R, Balducci J, Jones K. Does combination pharmacological and mechanical pre‐induction cervical ripening improve ripening to delivery interval?. American Journal of Obstetrics and Gynecology 1999;182(1):S136.
Sabra 2000 {published data only}
-
- Sabra A, Abdel‐Aleem H, Abdel‐Aleem A, Shaheen A. Misoprostol versus oxytocin safety and efficacy in induction of labor. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 1). 2000:95‐6.
Sanchez Ramos 1993 {published data only}
-
- Sanchez Ramos L, Kaunitz AM, Valle GO, Delke I, Schroeder PA, Briones DK. Labor induction with the prostaglandin E1 methyl analogue misoprostol versus oxytocin: a randomized trial. Obstetrics & Gynecology 1993;81:332‐6. - PubMed
-
- Sanchez‐Ramos L, Kaunitz AM, Valle GO, Delke I, Schroeder PA, Briones DK. Labor induction with the prostaglandin E1 methyl analog misoprostol vs oxytocin: a randomized trial. International Journal of Gynecology & Obstetrics 1993;43:229. - PubMed
Sanchez Ramos 2002 {published data only}
-
- Sanchez‐Ramos L, Danner CJ, Delke I, Kaunitz AM. The effect of tablet moistening on labor induction with intravaginal misoprostol: a randomized trial. Obstetrics & Gynecology 2002;99(6):1080‐4. - PubMed
Sharma 2005 {published data only}
-
- Sharma Y, Kumar S, Mittal S, Misra R, Dadhwal V. Evaluation of glyceryl trinitrate, misoprostol, and prostaglandin E2 gel for preinduction cervical ripening in term pregnancy. Journal of Obstetric and Gynaecology Research 2005;31(3):210‐5. - PubMed
Sheela 2006 {published data only}
-
- Sheela SR, Swamy MN, Ambika V. Induction of labour with 25 mcg versus 100 mcg of misoprostol. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:54.
Shen 2003 {published data only}
-
- Shen JX. Clinical study of labour induced by low dose misoprostol in prolonged pregnancy. Chinese Journal of Today's Medicine 2003;3(8):42‐3.
Shi 2003a {published data only}
-
- Shi XH, Shang LP, Tang LP. Clinical study of labour induced by low dose misoprostol. Journal Yiyang Medical College 2003;22(5):294‐5.
Shi 2003b {published data only}
-
- Shi XH, Ding AY, Zhang LP. Clinical study of labour induced by low dose misoprostol. Journal Yiyang Medical College 2003;22(5)(5):294‐5.
Su 1998 {published data only}
-
- Su MM, Shen YX. Clinical study of labour induced by low dose misoprostol in late gestation. Journal of Ningbo Medicine 1998;10(6):270.
Su 2003 {published data only}
-
- Su FM, Yang B, Xu HL, Zhang XM. A comparison between the oral and vaginal administration of misoprostol for labour induction at term. Journal of Xianning College (Medical sciences) 2003;17(1):43‐6.
Thach 2000 {published data only}
-
- Thach TS, Jamulitrat S, Chongsuviwatong V, Geater A, Pham TD. Misoprostol: an effective alternative to oxytocin for labour induction in term premature rupture of membrane and unfavourable cervix. XVI FIGO World Congress of Obstetrics and Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 3). 2000:53.
Tian 2003 {published data only}
-
- Tian LJ, Li YG, Xiao J, Wu SF. Clinical study of labour induced by low dose misoprostol and oxytocin. Chinese Journal of Today's Medicine 2003;3(10):27‐8.
Toppozada 1997 {published data only}
-
- Toppozada MK, Anwar MY, Hassan HA, el‐Gazaerly WS. Oral or vaginal misoprostol for induction of labor. International Journal of Gynecology & Obstetrics 1997;56:135‐9. - PubMed
Varaklis 1994 {published data only}
-
- Varaklis K, Cuming R, Stubblefield P. Misoprostol: a prostaglandin E1 analogue. International Journal of Gynecology & Obstetrics 1994;46:105.
Wang 1997a {published data only}
-
- Wang L, Shi C, Yang GZ, Li YY. A comparison of misoprostol and ricinus oil meal for cervical ripening and labor induction. Chung Hua Fu Chan Ko Tsa Chih 1997;32(11):666‐8. - PubMed
Wang 1997b {published data only}
-
- Wang Z, Li W, Ouyang W, Ding Y, Wang F, Xu L, et al. Cervical ripening in the third trimester of pregnancy with intravaginal misoprostol: a double‐blind, randomized, placebo‐controlled study. Journal of Tongji Medical University 1998;18(3):183‐6. - PubMed
-
- Wang Z, Li W, Ouyang W, Ding Y, Wang F, Xu L, et al. Safety and efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy. Chung Hua Fu Chan Ko Tsa Chih 1997;32(6):326‐8. - PubMed
Wang 1997c {published data only}
-
- Wang XG. Clinical study of labour induced by low dose misoprostol ‐ 83 cases. Journal of Jiangxi Medical College 1997;4:2009.
Wang 2000 {published data only}
-
- Wang YX, Zheng YZ, Yang L. Clinical study of labour induced by low dose misoprostol. World Journal of Medicine Today 2000;1(4):335‐6.
Wang 2005 {published data only}
-
- Wang ZH. Clinical study of labour induced by low dose misoprostol in late gestation. Chinese Medicine Hygiene 2005;6(14):32‐3.
Wicker 1995 {published data only}
-
- Wicker R, Albert J, Laurent S, Bellitt P. Evaluation of misoprostol and dinoprostone in cervical ripening. American Journal of Obstetrics and Gynecology 1995;172:424.
Wilk 2001 {published data only}
-
- Wilk M, Jureczko T, Preba R, Spinski A. Misoprostol versus oxytocin in induction of labor in post‐term pregnancy ‐ safety and effectiveness comparison. Wiadomosci Lekarskie 2001;54(11‐12):662‐7. - PubMed
Windrim 1997 {published data only}
-
- Windrim R, Bennett K, Mundle W, Young DC. Oral administration of misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 1997;89:392‐7. - PubMed
Wing 1999 {published data only}
-
- Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol to vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. - PubMed
Yang 2000 {published data only}
-
- Yang R. Clinical study of labour induced by low dose misoprostol in late gestation. Continuing Medical Education 2000;14(2):43‐4.
Young 2001 {published data only}
-
- Young D, Delaney T, Armson T, Fanning C. Lower dose vaginal and oral misoprostol in labor induction ‐ rct [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203.
Zang 1997 {published data only}
-
- Zhang LJ, Qu LP, Guo WX, Liu ZH. Clinical study of labour induced by low dose misoprostol. Journal of Ningxia Medicine 1997;19(6):362.
Zang 2003 {published data only}
-
- Zang CY. Clinical study of different doses of misoprostol for the induction of labour. Chinese Clinical Medicine Journal 2003;59:9839‐40.
Zhao 2003 {published data only}
-
- Zhao YL. Clinical study of labour induced by low dose misoprostol in late gestation. China Clinical Medicine Research Journal 2003;81:134‐42.
Zhu 1998 {published data only}
-
- Zhu HX, Hu LY, Luo Y, Li XQ. Clinical study on labour induction by low dose misoprostol. Jiang Shu Clinical Medical Journal 1998;2(3):255‐6.
Zhuang 2000 {published data only}
-
- Zhuang G, Su Q, Zhang J, Xie J, Zhu J, Cheng L. Study on misoprostol suspension per os for inducing term labor. Chinese Journal of Obstetric and Gynaecological Practice 2000;16(8):481‐3.
References to studies awaiting assessment
Abedi‐Asl 2007 {published data only}
-
- Abedi‐Asl Z, Farrokhi M, Rajaee M. Comparative efficacy of misoprostol and oxytocin as labor preinduction agents: a prospective randomized trial. Acta Medica Iranica 2007;45(6):443‐8.
Ayaz 2010 {published data only}
-
- Ayaz A, Shaukat S, Farooq MU, Mehmood K, Ahmad I, Ali Bahoo ML. Induction of labor: a comparative study of intravaginal misoprostol and dinoprostone. Taiwanese Journal of Obstetrics & Gynecology 2010;49(2):151‐5. - PubMed
Balci 2010 {published data only}
-
- Balci O, Mahmoud AS, Ozdemir S, Acar A. Induction of labor with vaginal misoprostol plus oxytocin versus oxytocin alone. International Journal of Gynecology & Obstetrics 2010;110(1):64‐7. - PubMed
Balci 2011 {published data only}
-
- Balci O, Mahmoud AS, Acar A, Colakoglu MC. Comparison of induction of labor with vaginal misoprostol plus oxytocin versus oxytocin alone in term primigravidae. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(9):1084‐7. - PubMed
Bebbington 2003a {published data only}
-
- Bebbington M, Pevzner L, Schmuel E, Bernstein P, Dayal A, Barnhard J, et al. Uterine tachysystole and hyperstimulation during induction of labor [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S211.
Bebbington 2003b {published data only}
-
- Bebbington M, Schmuel E, Pevzner L, Bernstein P, Dayal A, Barnhard J, et al. Misoprostol versus dinoprostone for labor induction at term: a randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S211.
Begum 2009 {published data only}
-
- Begum A, Butt RM, Kouser S, Naqvi S. Comparison of dinoprostone (pge2) and misoprostol (pge1) for induction of labor. Journal of Allama Iqbal Medical College 2009;7(4):20‐4.
Bolnick 2002b {published data only}
-
- Bolnick JM, Velazquez MD, Gonzalez JL, Rappaport VJ, McIlwain‐Dunivan G, Rayburn WF. Randomized trial between two active labor management protocols in the presence of an unfavorable cervix. American Journal of Obstetrics and Gynecology 2004;190(1):124‐8. - PubMed
Brennan 2011 {published data only}
-
- Brennan MC, Pevzner L, Wing DA, Powers BL, Rayburn WF. Retention of dinoprostone vaginal insert beyond 12 hours for induction of labor. American Journal of Perinatology 2011;28(6):479‐84. - PubMed
Bricker 2007 {published data only}
-
- Bricker L, Peden H, Alfirevic Z. The prommis trial: a multicentre randomised trial to evaluate a low dose misoprostol regimen for induction of labour in the presence of prelabour rupture of the amniotic membranes. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22‐S23.
Bricker 2008 {published data only}
-
- Bricker L, Peden H, Tomlinson AJ, Al‐Hussaini TK, Idama T, Candelier C, et al. Titrated low‐dose vaginal and/or oral misoprostol to induce labour for prelabour membrane rupture: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2008;115(12):1503‐11. - PubMed
Chaudhuri 2011 {published data only}
-
- Chaudhuri S, Mitra SN, Banerjee PK, Biswas PK, Bhattacharyya S. Comparison of vaginal misoprostol tablets and prostaglandin E2 gel for the induction of labor in premature rupture of membranes at term: a randomized comparative trial. Journal of Obstetrics and Gynaecology Research 2011;37(11):1564‐71. - PubMed
Chen 2000a {published data only}
-
- Chen TM. Clinical analysis of misoprostol on induction of labor in term pregnancy. Journal of Zhenjiang Medical College 2000, (4):652‐3.
Deng 1999 {published data only}
-
- Deng LL, Huang ZJ. Observation on the efficacy of intravaginal misoprostol for cervical ripening in the third trimester of pregnancy. Journal of Nursing Science 1999;14(2):67‐8.
ElSedeek 2009 {published data only}
-
- ElSedeek M, Awad EE, ElSebaey SM. Evaluation of postpartum blood loss after misoprostol‐induced labour. BJOG: an international journal of obstetrics and gynaecology 2009;116(3):431‐5. - PubMed
Ezechi 2008 {published data only}
-
- Ezechi OC, Loto OM, Ezeobi PM, Okogbo FO, Gbajabiamila T, Nwokoro CA. Safety and efficacy of misoprostol in induction of labour in prelabour rupture of fetal membrane in Nigerian women: a multicenter study. Iranian Journal of Reproductive Medicine 2008;6(2):83‐7.
Girija 2009 {published data only}
Girija 2011 {published data only}
-
- Girija S, Manjunath AP. A randomized controlled trial comparing low dose vaginal misoprostol and dinoprostone gel for labor induction. Journal of Obstetrics and Gynecology of India 2011;61(2):153‐60.
Gupta 2006 {published data only}
-
- Gupta N, Mishra SL, Shradha J. A randomized clinical trial comparing misoprostol and dinoprostone for cervical ripening and labor induction. Journal of Obstetrics and Gynecology of India 2006;56(2):149‐51.
Gupta 2010 {published data only}
-
- Gupta HP, Singh U, Mehrotra S. Comparative evaluation of 25 ug and 50ug of intravaginal misoprostol for induction of labor. Journal of Obstetrics and Gynecology of India 2010;60(1):51‐4.
Hosli 2008 {published data only}
-
- Hosli I, Zanetti‐Daellenbach R, Gairing A, Holzgreve W, Lapaire O. Selection of appropriate prostaglandin for the induction of labor at term is more predictive for the achievement of delivery within 24 hours than pre‐assessed cervical parameters ‐ a prospective, randomized trial. Geburtshilfe und Frauenheilkunde 2008;68(2):147‐51.
Joo 2000 {published data only}
-
- Joo SH, Hur EJ, Park JW, Lee WK. A comparison of the safety and efficacy of intravaginal prostaglandin e1 ( misoprostol ) and prostaglandin e2 ( dinoprostone ) to induce labor. Korean Journal of Obstetrics and Gynecology 2000;43(3):444‐50.
Kim 2000 {published data only}
-
- Kim JH, Yang HS. A comparison of intravaginal misoprostol and dinoprostone for cervical ripening and labor inducton in term pregnancy with unfavorable cervix. Korean Journal of Obstetrics and Gynecology 2000;43(2):243‐7.
Li 2000 {published data only}
-
- Li FM. A study of misoprostol on induction of labor in term pregnancy. Journal of Practical Obstetrics and Gynecology 2000;16(3):139‐41.
Lughmani 2009 {published data only}
-
- Lughmani S. Vaginal misoprostol versus oxytocin infusion for labour induction in great grand multipara. A randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S250.
Mahendru 2011 {published data only}
Milchev 2003 {published data only}
-
- Milchev N, Kuzmanov B, Terzhumanov R. Cytotec: an effective drug for the induction of labor [Cytotec: efektivno sredstvo za induktsiia na razhdaneto]. Akusherstvo i Ginekologiia 2003;42(3):9‐11. - PubMed
Moodley 2003 {published data only}
-
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Journal of Obstetrics and Gynaecology 2003;9(2):34‐7. - PubMed
Nigam 2010 {published data only}
-
- Nigam A, Madan M, Puri M, Agarwal S, Trivedi SS. Labour induction with 25 micrograms versus 50 micrograms intravaginal misoprostol in full term pregnancies. Tropical Doctor 2010;40(1):53‐5. - PubMed
Norzilawati 2010 {published data only}
-
- Norzilawati MN, Mashita MK, Shuhaila A, Zaleha AM. Vaginal misoprostol versus dinoprostone for induction of labor. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):244.
Ozkan 2009 {published data only}
-
- Ozkan S, Caliskan E, Doger E, Yucesoy I, Ozeren S, Vural B. Comparative efficacy and safety of vaginal misoprostol versus dinoprostone vaginal insert in labor induction at term: a randomized trial. Archives of Gynecology and Obstetrics 2009;280(1):19‐24. - PubMed
Pevzner 2008 {published data only}
-
- Pevzner L, Rumney P, Petersen R, Wing D. Predicting a successful induction of labor: a secondary analysis of misoprostol vaginal insert trial. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S72.
Pevzner 2009a {published data only}
-
- Pevzner L, Alfirevic Z, Powers B, Wing D. Cardiotocographic abnormalities associated with misoprostol and dinoprostone cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S124. - PubMed
Pevzner 2009b {published data only}
-
- Pevzner L, Rayburn WF, Rumney P, Wing DA. Factors predicting successful labor induction with dinoprostone and misoprostol vaginal inserts. Obstetrics & Gynecology 2009;114(2 Pt 1):261‐7. - PubMed
Pevzner 2011 {published data only}
-
- Pevzner L, Alfirevic Z, Powers BL, Wing DA. Cardiotocographic abnormalities associated with misoprostol and dinoprostone cervical ripening and labor induction. European Journal of Obstetrics & Gynecology and Reproductive Biology 2011;156(2):144‐8. - PubMed
Pezvner 2011 {published data only}
-
- Pezvner L, Powers BL, Wing DA. Factors predicting successful induction of labor with misoprostol vaginal insert. Reproductive Sciences 2011;18(3 Suppl 1):182A‐3A.
Powers 2011 {published data only}
-
- Powers B, Parker L, Miller H, Wing DA, Rayburn W. A double‐blind, randomized, multicenter, dose‐ranging phase II study of the misoprostol vaginal insert. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S48.
Prager 2008 {published data only}
-
- Prager M, Eneroth‐Grimfors E, Edlund M, Marions L. A randomised controlled trial of intravaginal dinoprostone, intravaginal misoprostol and transcervical balloon catheter for labour induction. BJOG: an international journal of obstetrics and gynaecology 2008;115(11):1443‐50. - PubMed
Rolland 2011 {published data only}
-
- Rolland Souza A. [Titrated oral suspension compared with vaginal misoprostol for labor induction: a randomized controlled trial] [Misoprostol em solução oral titulada escalonada versus via vaginal para indução do trabalho de parto: ensaio clínico randomizado]. Revista Brazileira de Ginecologia e Obstetricia 2011;33(9):270.
Saeed 2011 {published data only}
-
- Saeed GA, Fakhar S, Nisar N, Alam AY. Misoprostol for term labor induction: a randomized controlled trial. Taiwanese Journal of Obstetrics & Gynecology 2011;50(1):15‐9. - PubMed
Shakya 2010 {published data only}
-
- Shakya R, Shrestha J, Thapa P. Safety and efficacy of misoprostol and dinoprostone as cervical ripening agents. JNMA, Journal of the Nepal Medical Association 2010;49(177):33‐7. - PubMed
Shanmugham 2011 {published data only}
-
- Shanmugham D, Behera AK. Comparison of prostaglandin E1 (misoprostol) with prostaglandin E2 (Cerviprime gel) for induction of labour in primigravidae. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:112.
Stephenson 2011 {published data only}
-
- Stephenson ML, Pevzner L, Powers BL, Wing DA. Race/ethnicity differences in labor outcomes with misoprostol and dinoprostone vaginal inserts. Reproductive Sciences 2011;18(3 Suppl 1):183A.
Tabasi 2007 {published data only}
-
- Tabasi Z, Behrashi M, Mahdian M. Vaginal misoprostol versus high dose of oxytocin for labor induction: a comparative study. Pakistan Journal of Biological Sciences 2007;10(6):920‐3. - PubMed
Tan 2010 {published data only}
-
- Tan TC, Yan SY, Chua TM, Biswas A, Chong YS. A randomised controlled trial of low‐dose misoprostol and dinoprostone vaginal pessaries for cervical priming. BJOG: an international journal of obstetrics & gynaecology 2010;117(10):1270‐7. - PubMed
Wang 2000 {published data only}
-
- Wang YZ, Zhu BL, Li M, Bu YG. Clinical observation of the effects of accelerating the maturation of the cervix and induction by various doses of misoprostol. Anhui Medical Journal 2000;21(2):23‐4.
Wing 2008 {published data only}
-
- Wing DA, for the Misoprostol Vaginal Insert Consortium. Misoprostol vaginal insert compared with dinoprostone vaginal insert: a randomized controlled trial. Obstetrics & Gynecology 2008;112(4):801‐12. - PubMed
Wing 2011 {published data only}
-
- Wing DA, Miller H, Parker L, Powers BL, Rayburn WF, for theMVIM‐O‐204I. Misoprostol vaginal insert for successful labor induction. A randomized controlled trial. Obstetrics & Gynecology 2011;117(3):533‐41. - PubMed
Yang 2000a {published data only}
-
- Yang Z, Wang YL. A comparison of misoprostol and prostaglandin e2 gel for cervical ripening and induction of labour: a clinical study on efficacy of two different dosages gemfibrozil administered in hyperlipidemic patients. Journal of Jinzhou Medical College 2000;21(1):10‐2.
Yin 2006 {published data only}
-
- Yin CY, Zhou JZ, Wang BP, Lu XY. Effect and risk analysis of misoprostol in stimulating cervical maturity for post‐term pregnancy. Nan Fang Yi Ke Da Xue Xue Bao//Journal of Southern Medical University 2006;26(2):182‐4; 188. - PubMed
References to ongoing studies
Botero 1998 {unpublished data only}
-
- Botero L. Labor induction with unfavourable cervix. Randomized controlled trial. Double blind method. Oxytocin versus misoprostol. Personal communication 1998.
Gregson 2003 {published data only}
-
- Gregson S. To compare the safety and efficacy of 'low dose' vaginal misoprostol and dinoprostone vaginal gel for induction of labour at term. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 15 September 2004).
Jackson 2000 {published data only}
-
- Jackson N, Paterson‐Brown S. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of the membranes [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1181‐2. - PubMed
Additional references
Alfirevic 2006
Bennett 1997
-
- Bennett BB. Uterine rupture during induction of labor at term with intravaginal misoprostol. Obstetrics & Gynecology 1997;89(5 Pt 2):832‐3. - PubMed
Bishop 1964
-
- Bishop EH. Pelvic scoring for elective induction. Obstetrics & Gynecology 1964;24:266‐8. - PubMed
Blanchette 1999
-
- Blanchette HA, Nayak S, Erasmus S. Comparison of the safety and efficacy of intravaginal misoprostol (prostaglandin E1) with those of dinoprostone (prostaglandin E2) for cervical ripening and induction of labor in a community hospital. American Journal of Obstetrics and Gynecology 1999;180:1551‐9. - PubMed
Boulvain 2001
Boulvain 2005
Boulvain 2008
Bricker 2000
Choy‐Hee 2001
-
- Choy‐Hee L, Raynor BD. Misoprostol induction of labor among women with a history of cesarean delivery. American Journal of Obstetrics and Gynecology 2001;184:1115‐7. - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1 Oxford, England: The Cochrane Collaboration, 2000.
Costa 1993
-
- Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet 1993;341:1258‐61. - PubMed
Curtis 1987
-
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. - PubMed
Daisley 2000
-
- Daisley H. Maternal mortality following the use of misoprostol. Medicine, Science and the Law 2000;40(1):78‐82. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001.
Egarter 1990
-
- Egarter CH, Husslein PW, Rayburn WF. Uterine hyperstimulation after low‐dose prostaglandin E2 therapy: tocolytic treatment in 181 cases. American Journal of Obstetrics and Gynecology 1990;163:794‐6. - PubMed
Fonseca 1991
-
- Fonseca W, Alencar AJC, Mota FSB, Coelho HLL. Misoprostol and congenital malformations. Lancet 1991;338:56. - PubMed
French 2001
Gherman 1999
-
- Gherman RB, McBrayer S, Browning J. Uterine rupture associated with vaginal birth after cesarean section: a complication of intravaginal misoprostol?. Gynecologic and Obstetric Investigation 2000;50:212‐3. - PubMed
Hapangama 2009
Higgins 2008a
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2008b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008.. Available from www.cochrane‐handbook.org.
Hill 2000
-
- Hill DA, Chez RA, Quinlan J, Fuentes A, LaCombe J. Uterine rupture and dehiscence associated with intravaginal misoprostol cervical ripening. Journal of Reproductive Medicine 2000;45:823‐6. - PubMed
Hofmeyr 2009
-
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] - DOI
Howarth 2001
Hutton 2001
Kavanagh 2001
Kavanagh 2005
Kavanagh 2006a
Kavanagh 2006b
Keirse 1993
-
- Keirse MJNC. Prostaglandins in preinduction cervical ripening: meta‐analysis of worldwide clinical experience. Journal of Reproductive Medicine 1993;38 Suppl:89‐98. - PubMed
Kelly 2001a
Kelly 2001b
Kelly 2001c
Kelly 2003
Kelly 2008
-
- Kelly AJ, Kavanagh J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006901] - DOI
Khosla 2002
-
- Khosla AH, Sirohiwal D, Sangwan K. A still birth and uterine rupture during induction of labour with oral misoprostol. Australia and New Zealand Journal of Obstetrics and Gynaecology 2002;42(4):412. - PubMed
Luckas 2000
MacKenzie 1997
-
- MacKenzie IZ, Burns E. Randomised trial of one versus two doses of prostaglandin E2 for induction of labour: 1. Clinical outcome. British Journal of Obstetrics and Gynaecology 1997;104:1062‐7. - PubMed
Mariani Neto 1987
-
- Mariani Neto C, Leao EJ, Baretto EM, Kenj G, Aquino MM. Use of misoprostol for labour induction in stillbirths. Revista Paulista de Medicina 1987;105:325‐8. - PubMed
Mariani Neto 1988
-
- Mariani Neto C, Delbin AL, Val RD. Tocographic pattern caused by misoprostol [Padrao tocografico desencadeado pelo misoprostol]. Revista Paulista de Medicina 1988;106:205‐8. - PubMed
Matonhodze 2002
-
- Matonhodze BB, Katsoulis LC, Hofmeyr GJ. Labor induction and meconium: in vitro effects of oxytocin, dinoprostone and misoprostol on rat ileum relative to myometrium. Journal of Perinatal Medicine 2002;30(5):405‐10. - PubMed
Matthews 1999
-
- Mathews JE, Mathai M, George A. Uterine rupture in a multiparous woman during labor induction with oral misoprostol. American Journal of Obstetrics and Gynecology 1999;181(3):626‐9. - PubMed
Merrell 1995
-
- Merell DA, Koch MAT. Induction of labour with misoprostol in the second and third trimesters of pregnancy. South African Medical Journal 1995;85:1088‐90. - PubMed
Merrell 1996
-
- Merrell DA, Koch MAT, Thomas PC. Experience with vaginal and rectal misoprostol as an oxytocic agent in pregnancy.. PAFMACH Conference; 1996; Johannesburg, South Africa. 1996.
Mitri 1987
-
- Mitri F, Hofmeyr GJ, Gelderen CJ. Meconium during labour: self‐medication and other associations. South African Medical Journal 1987;71:431‐3. - PubMed
Muzonzini 2004
Norman 1991
-
- Norman JE, Thong KJ, Baird DT. Uterine contractility and induction of abortion in early pregnancy by misoprostol and mifepristone. Lancet 1991;338:1233‐6. - PubMed
Plaut 1999
-
- Plaut MM, Schwartz ML, Lubarsky SL. Uterine rupture associated with the use of misoprostol in the gravid patient with a previous cesarean section. American Journal of Obstetrics and Gynecology 1999;180(6 pt 1):1532‐42. - PubMed
Ravasia 2000
-
- Ravasia DJ, Wood SL, Pollard JK. Uterine rupture during induced trial of labor among women with previous cesarean delivery. American Journal of Obstetrics and Gynecology 2000;183:1176‐9. - PubMed
RevMan 2008 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sanchez‐Ramos 1997
-
- Sanchez‐Ramos L, Kaunitz AM, Wears RL, Delke I, Gaudier FL. Misoprostol for cervical ripening and labor induction: a meta‐analysis. Obstetrics & Gynecology 1997;89:633‐42. - PubMed
Sciscione 1998
-
- Sciscione AC, Nguyen L, Manley JS, Shlossman PA, Colmorgen GH. Uterine rupture during preinduction cervical ripening with misoprostol in a patient with a previous caesarean delivery. Australian and New Zealand Journal of Obstetrics and Gynaecology 1998;38(1):96‐7. - PubMed
Senior 1993
Smith 2003
Smith 2004
Thomas 2001
Wing 1999b
-
- Wing DA. Labor induction with misoprostol. American Journal of Obstetrics and Gynecology 1999;181(2):339‐45. - PubMed
Zieman 1997
-
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. - PubMed
References to other published versions of this review
CDSR 1999
-
- Hofmeyr GJ. Misoprostol administered vaginally for cervical ripening and labour induction in the third trimester (Cochrane Review). Cochrane Database of Systematic Reviews 1998, Issue 3. - PubMed
CDSR 2000
-
- Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and labour induction in late pregnancy (Cochrane Review). Cochrane Database of Systematic Reviews 2000, Issue 4. - PubMed
CDSR 2004
-
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour (Cochrane Review). Cochrane Database of Systematic Reviews 2004, Issue 1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

