Finasteride for benign prostatic hyperplasia
- PMID: 20927745
- PMCID: PMC8908761
- DOI: 10.1002/14651858.CD006015.pub3
Finasteride for benign prostatic hyperplasia
Abstract
Background: Benign prostatic hyperplasia (BPH), a non-malignant enlargement of the prostate in aging men, can cause bothersome urinary symptoms (intermittency, weak stream, straining, urgency, frequency, incomplete emptying). Finasteride, a five-alpha reductase inhibitor (5ARI), blocks the conversion of testosterone to dihydrotestosterone, reduces prostate size, and is commonly used to treat symptoms associated with BPH.
Objectives: To compare the clinical effectiveness and harms of finasteride versus placebo and active controls in the treatment of lower urinary tract symptoms (LUTS).
Search strategy: We searched The Cochrane Library (which includes CDSR (Cochrane Database of Systematic Reviews), DARE (Database of Abstracts of Reviews of Effects), HTA (Heath Technology Assessments), and CENTRAL (Cochrane Central Register of Controlled Trials, and which includes EMBASE and MEDLINE), LILACS (Latin American and Caribbean Center on Health Sciences Information) and Google Scholar for randomized, controlled trials (RCTs). We also handsearched systematic reviews, references, and clinical-practice guidelines.
Selection criteria: Randomized trials in the English language with placebo and/or active arms with a duration of at least 6 months.
Data collection and analysis: JT extracted the data, which included patient characteristics, outcomes, and harms. Our primary outcome was change in a validated, urinary symptom-scale score, such as the AUA/IPSS. A clinically meaningful change was defined as 4 points. We also categorized outcomes by trial lengths of ≤ 1 year (short term) and > 1 year (long term).
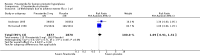
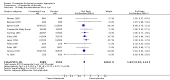
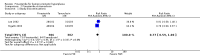
Main results: Finasteride consistently improved urinary symptom scores more than placebo in trials of > 1 year duration, and significantly lowered the risk of BPH progression (acute urinary retention, risk of surgical intervention, ≥ 4 point increase in the AUASI/IPSS). In comparison to alpha-blocker monotherapy, finasteride was less effective than either doxazosin or terazosin, but equally effective compared to tamsulosin. Both doxazosin and terazosin were significantly more likely than finasteride to improve peak urine flow and nocturia, versus finasteride. Versus tamsulosin, peak urine flow and QoL improved equally well versus finasteride. However, finasteride was associated with a lower risk of surgical intervention compared to doxazosin, but not to terazosin, while finasteride and doxazosin were no different for risk of acute urinary retention. Two small trials reported no difference in urinary symptom scores between finasteride and tamsulosin. Finasteride + doxazosin and doxazosin monotherapy improved urinary symptoms equally well (≥ 4 point improvement).For finasteride, there was an increased risk of ejaculation disorder, impotence, and lowered libido, versus placebo. Versus doxazosin, finasteride had a lower risk of asthenia, dizziness, and postural hypotension, and versus terazosin, finasteride had a significant, lower risk of asthenia, dizziness, and postural hypotension.
Authors' conclusions: Finasteride improves long-term urinary symptoms versus placebo, but is less effective than doxazosin. Long-term combination therapy with alpha blockers (doxazosin, terazosin) improves symptoms significantly better than finasteride monotherapy. Finasteride + doxazosin improves symptoms equally - and clinically - to doxazosin alone. In comparison to doxazosin, finasteride + doxazosin appears to improve urinary symptoms only in men with medium (25 to < 40 mL) or large prostates (≥ 40 mL), but not in men with small prostates (25 mL).Comparing short to long-term therapy, finasteride does not improve symptoms significantly better than placebo at the short term, but in the long term it does, although the magnitude of differences was very small (from < 1.0 point to 2.2 points). Doxazosin improves symptoms better than finasteride both short and long term, with the magnitude of differences ∼2.0 points and 1.0 point, respectively. Finasteride + doxazosin improves scores versus finasteride alone at both short and long term, with mean differences ∼2.0 points for both time points. Finasteride + doxazosin versus doxazosin improves scores equally for short and long term.Drug-related adverse effects for finasteride are rare; nevertheless, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder, versus placebo. Versus doxazosin, which has higher rates of dizziness, postural hypotension, and asthenia, men taking finasteride are at increased risk for impotence, erectile dysfunction, decreased libido, and ejaculation disorder. Finasteride significantly reduces asthenia, postural hypotension, and dizziness versus terazosin. Finasteride significantly lowers the risk of asthenia, dizziness, ejaculation disorder, and postural hypotension, versus finasteride + terazosin.
Conflict of interest statement
None reported.
Figures




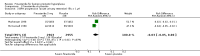




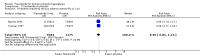
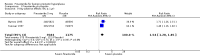



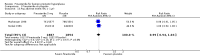






Update of
References
References to studies included in this review
Abrams 1999 {published data only}
-
- Abrams P, Schafer W, Tammela TLJ, Barrett DM, Hedlund H, Rollema HJ, et al. Improvement of pressure flow parameters with finasteride is greater in men with large prostates. The Journal of Urology 1999;161(5):1513‐7. - PubMed
Agrawal 2001 {published data only}
-
- Agrawal MS, Aron M. Prospective double‐blind randomized controlled trial of terazosin, finasteride and allylestrenol in the management of benign prostatic hyperplasia. Indian Journal of Urology [serial online] 2001 [cited 2010 Mar 4]; Vol. 17, issue 2:132‐40. Available from: http://www.indianjurol.com/text.asp$2001/17/2/132/21043.
Andersen 1995 {published data only}
-
- Andersen JT, Ekman P, Wolf H, Beisland HO, Johansson JE, Kontturi M, Lehtonen T, Tveter K, and The Scandinavian BPH Study Group. Can Finasteride reverse the progress of benign prostatic hyperplasia? A two‐year placebo‐controlled study. Urology 1995;46(5):631‐7. - PubMed
Beisland 1992 {published data only}
-
- Beisland HO, Binkowitz B, Brekkan E, Ekman P, Kontturi M, Letonen T, et al. Scandinavian clinical study of finasteride in the treatment of benign prostatic hyperplasia. European Urology 1992;22:271‐7. - PubMed
Byrnes 1995 {published data only}
-
- Byrnes CA, Morton AS, Liss CL, Lippert MC, Gillenwater JY on behalf of the CUSP investigators. Efficacy, tolerability, and effect on health‐related quality of life of finasteride versus placebo in men with symptomatic benign prostatic hyperplasia: a community‐based study. Clinical Therapeutics 1995;17(5):956‐69. - PubMed
Carraro 1996 {published data only}
-
- Carraro J‐C, Raynaud J‐P, Koch G, Chisholm GD, Silverio F, Teillac P, et al. Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: a randomized international study of 1,098 patients. The Prostate 1996;29:231‐40. - PubMed
Finasteride Study Group {published data only}
-
- The Finasteride Study Group. Finasteride (MK‐906) in the treatment of benign prostatic hyperplasia. The Prostate 1993;22:291‐9. - PubMed
Gormley 1992 {published data only}
-
- Gormley GJ, Stoner E, Bruskewitz RC, Imperato‐McGinley J, Walsh PC, McConnell JD, et al. The effect of finasteride in men with benign prostatic hyperplasia. The New England Journal of Medicine 1992;327(17):1185‐91. - PubMed
Kirby 2003 {published data only}
-
- Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, Sweeney M, and Grossman EB for the PREDICT Study Investigators. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology 2003;61:119‐26. - PubMed
Lee 2002 {published data only}
-
- Lee E. Comparison of tamsulosin and finasteride for lower urinary tract symptoms associated with benign prostatic hyperplasia in Korean patients. The Journal of International Medical Research 2002;30:584‐90. - PubMed
Lepor 1996 {published data only}
-
- Johnson, II TM, Jones K, Williford WO, Kutner MH, Issa MM, Lepor H. Changes in nocturia from medical treatment of benign prostatic hyperplasia: secondary analysis of the Department of Veterans Affairs Cooperative Study Trial. The Journal of Urology 2003;170:145‐8. - PubMed
-
- Lepor H, Jones K, Williford W. The mechanism of adverse events associated with terazosin: an analysis of the Veterans Affairs Cooperative Study. The Journal of Urology 2000;163:1134‐7. - PubMed
-
- Lepor H, Williford WO, Barry MJ, Brawer MK, Dixon CM, Gormley G, Haakenson C, Machi M, Narayan P, Padley RJ, for the Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. The efficacy of terazosin, finasteride, or both n benign prostatic hyperplasia. The New England Journal of Medicine 1996;335(8):533‐9. - PubMed
-
- Lepor H, Williford WO, Barry MJ, Haakenson C, Jones K. The impact of medical therapy on bother due to symptoms, quality of life and global outcome, and factors predicting response. Journal of Urology 1998;160(4):1358‐67. - PubMed
Marberger 1998 {published data only}
-
- Marberger MJ, on behalf of the PROWESS Study Group. Long‐term effects of finasteride in patients with benign prostatic hyperplasia: a double‐blind, placebo‐controlled, multicenter study. Urology 1998;51:677‐86. - PubMed
Marks 1997 {published data only}
-
- Marks LS, Partin AW, Gormley GJ, Dorey FJ, Shery ED, Garris JB, et al. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. The Journal of Urology 1997;157(6):2171‐8. - PubMed
McConnell 1998 {published data only}
-
- Kaplan S, Garvin D, Gilhooly P, Koppel M, Labasky R, Milsten R, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia‐related outcomes and long‐term response to finasteride. Urology 2000;56:610‐6. - PubMed
-
- Kaplan SA, Holtgrewe HL, Bruskewitz R, Saltzman B, Mobley D, Narayan P, et al. Comparison of the efficacy and safety of finasteride in older versus younger men with benign prostatic hyperplasia. Urology 2001;57:1073‐7. - PubMed
-
- McConnell JD, Bruskewitz R, Walsh P, Andriole G, Lieber M, Holtgrewe HL, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. New England Journal of Medicine 1998;338(9):557‐63. - PubMed
-
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo‐treated men with benign prostatic hyperplasia. Urology 2003;61:579‐84. - PubMed
McConnell 2003 {published data only}
-
- Johnson, II TM, Burrows PK, Kusek JW, Nyberg LM, Tenover JL, Lepor H, et al. The effect of doxazosin, finasteride and combination therapy on nocturia in men with benign prostatic hyperplasia. The Journal of Urology 2007;178:2045‐51. - PubMed
-
- Kaplan SA, McConnell JD, Roehrborn CG, Meehan AG, Lee MW, Noble WR, et al. Combination therapy with doxazosin and finasteride for benign prostatic hyperplasia in patients with lower urinary tract symptoms and a baseline total prostate volume of 25 Ml or greater. The Journal of Urology 2006;175:217‐21. - PubMed
-
- McConnell JD, Roehrborn CG, Bautista OM, Andriole, Jr GL, Dixon CM, Kusek JW, et al. The long‐term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. The New England Journal of Medicine 2003;349(25):2387‐98. - PubMed
Nickel 1996 {published data only}
Polat 1997 {published data only}
-
- Polat Ő, Őzbey I, Gűl O, Demirel A, Bayraktar Y. Pharmacotherapy of benign prostatic hyperplasia: inhibitor of 5 Alpha‐reductase. International Urology and Nephrology 1997;293(3):323‐30. - PubMed
Rigatti 2003 {published data only}
-
- Rigatti P, Brausi M, Scarpa RM, Porru D, Schumacher H, and Rizzi CA for the MICTUS Study Group. A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 2003;6:315‐23. - PubMed
Sökeland 2000 {published data only}
-
- Sökeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. British Journal of Urology International 2000;86:439‐42. - PubMed
Tammela 1993 {published data only}
-
- Tammela TLJ, Kontturi MJ. Urodynamic effects of finasteride in the treatment of bladder outlet obstruction due to benign prostatic hyperplasia. The Journal of Urology 1993;149:342‐4. - PubMed
Tempany 1993 {published data only}
-
- Tempany CMC, Partin AW, Zerhouni EA, Zinreich SJ, Walsh PC. The influence of finasteride on the volume of the peripheral and periurethral zones of the prostate in men with benign prostatic hyperplasia. The Prostate 1993;22:39‐42. - PubMed
Tenover 1997 {published data only}
-
- Tenover JL, Pagano GA, Morton AS, Liss CL, Byrnes CA, on behalf of the Primary Care Investigator Study Group. Efficacy and tolerability of finasteride in symptomatic benign prostatic hyperplasia: a primary care study. Clinical Therapeutics 1997;19(2):243‐58. - PubMed
Yu 1995 {published data only}
-
- Yu H‐J, Chiu T‐Y, Lai M‐K. Therapeutic effects of finasteride in benign prostatic hyperplasia: a randomized double‐blind controlled trial. Journal of the Formosan Medical Association 1995;94(1/2):37‐41. - PubMed
References to studies excluded from this review
Andriole 1998 {published data only}
-
- Andriole GL, Guess HA, Epstein JI, Wise H, Kadmon D, Crawford ED, et al. Treatment with finasteride preserves usefulness of prostate‐specific antigen in the detection of prostate cancer: results of a randomized, double‐blind, placebo‐controlled clinical trial. Urology 1998;52:195‐202. - PubMed
Baldwin 2001 {published data only}
-
- Baldwin KC, Ginsberg PC, Roehrborn CG, Harkaway RC. Discontinuation of alpha‐blockade after initial treatment with finasteride and doxazosin in men with lower urinary tract symptoms and clinical evidence of benign prostatic hyperplasia. Urology 2001;58:203‐9. - PubMed
Bruskewitz 1999 {published data only}
-
- Bruskewitz R, Girman CJ. Effect of finasteride on bother and health‐related quality of life associated with benign prostatic hyperplasia. The Journal of Urology 1999;161(4S Supplement):268. - PubMed
Ekman 1995 {published data only}
-
- Ekman P. Endocrine therapy for benign prostatic hyperplasia. Journal d'Urologie 1995;101(1):22‐5. - PubMed
Ekman 1996 {published data only}
-
- Ekman P, Andersen JT, Wolf H. Finasteride in the treatment of benign prostatic hyperplasia. Acta Urologica Belgica 1996;64(1):IX‐XII. - PubMed
Geller 1995 {published data only}
-
- Geller J. Five‐year follow‐up of patients with benign prostatic hyperplasia treated with finasteride. European Urology 1995;27:267‐73. - PubMed
Girman 1996 {published data only}
-
- Girman CJ, Kolman C, Liss CL, Bolognese JA, Binkowitz BS, Stoner E, Finasteride Study Group. Effects of finasteride on the health‐related quality of life in men with symptomatic benign prostatic hyperplasia. The Prostate 1996;29:83‐90. - PubMed
Gormley 1994 {published data only}
-
- Gormley GJ, Stoner E. Clinical results with finasteride. Progress in Clinical and Biological Research 1994;386:205‐7. - PubMed
Grino 1993 {published data only}
-
- Grino PB, Bruskewitz R, Blaivas JG, Siroky MB, Andersen JT, Cook T, Stoner E. Maximum urinary flow rate by uroflowmetry: automatic or visual interpretation. The Journal of Urology 1993;149:339‐41. - PubMed
Grino 1994 {published data only}
-
- Grino P, Stoner E, and the Finasteride Study Group. Finasteride for the treatment and control of benign prostatic hyperplasia: summary of Phase III controlled studies. European Urology 1994;25(suppl 1):24‐8. - PubMed
Jeong 2009 {published data only}
-
- Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5a‐reductase inhibitors on prostate volume and symptoms in men with BPH: A prospective study. Urology 2009;73:802‐6. - PubMed
Kaplan 2000 {published data only}
-
- Kaplan S, Garvin D, Gilhooly P, Koppel M, Labasky R, Milsten R, et al. Impact of baseline symptom severity on future risk of benign prostatic hyperplasia‐related outcomes and long‐term response to finasteride. Urology 2000;56:610‐6. - PubMed
Kaplan 2008 {published data only}
-
- Kaplan SA, Roehrborn CG, McConnell JD, Meehan AG, Surynawanshi S, Lee JY, et al. Long‐term treatment with finasteride results in a clinically significant reduction in total prostate volume compared to placebo over the full range of baseline prostate sizes in men enrolled in the MTOPS Trial. The Journal of Urology 2008;180:1030‐3. - PubMed
Kirby 1992 {published data only}
-
- Kirby RS, Bryan J, Eardley I, Christmas TJ, Liu S, Holmes SAV, et al. Finasteride in the treatment of benign prostatic hyperplasia. A dynamic evaluation. British Journal of Urology 1992;70:65‐72. - PubMed
Lam 2003 {published data only}
-
- Lam JS, Romas NA, Lowe FC. Long‐term treatment with finasteride in men with symptomatic benign prostatic hyperplasia: 10‐year follow‐up. Urology 2003;61:354‐58. - PubMed
Lowe 2003 {published data only}
-
- Lowe FC, McConnell JD, Hudson PB, Romas NA, Boake R, Lieber M, et al. Long‐term 6‐year experience with finasteride in patients with benign prostatic hyperplasia. Urology 2003;61:791‐6. - PubMed
Marberger 2000 {published data only}
-
- Marberger MJ, Andersen JT, Nickel JC, Malice M‐P, Gabriel M, Pappas F, et al. Prostate volume and serum prostate‐specific antigen as predictors of acute urinary retention. European Urology 2000;38:563‐8. - PubMed
Marks 1999 {published data only}
-
- Marks LS, Partin AW, Dorey FJ, Gormley GJ, Epstein JI, Garris JB, et al. Long‐term effects of finasteride on prostate tissue composition. Urology 1999;53:574‐80. - PubMed
Moore 1995 {published data only}
-
- Moore E, Bracken B, Bremner W, Geller J, Imperato‐McGinley J, McConnell J, et al. Proscar®: Five‐year experience. European Urology 1995;28:304‐9. - PubMed
Nacey 1995 {published data only}
-
- Nacey JN, Mefan PJ, Delahunt B. The effect of finasteride on prostate volume, urinary flow and symptom score in men with benign prostatic hyperplasia. Australia New Zealand Journal of Surgery 1995;65:35‐9. - PubMed
Paick 2005 {published data only}
-
- Paick SH, Meehan A, Lee M, Penson DF, Wessells H. The relationship among lower urinary tract symptoms, prostate specific antigen and erectile dysfunction in men with benign prostatic hyperplasia: results from the PROSCAR long‐term efficacy and safety study. The Journal of Urology 2005;173:903‐7. - PubMed
Perimenis 2002 {published data only}
-
- Perimenis P, Gyftopoulos K, Markou S, Barbalias G. Effects of finasteride and cyproterone acetate on hematuria associated with benign prostatic hyperplasia: a prospective, randomized, controlled study. Urology 2002;59:373‐7. - PubMed
Roehrborn 2000 {published data only}
-
- Roehrborn CG, Bruskewitz R, Nickel GC, Glickman S, Cox II C, Anderson R, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years. European Urology 2000;37:528‐36. - PubMed
Roehrborn 2000b {published data only}
-
- Roehrborn CG, McConnell J, Bonilla J, Rosenblatt S, Hudson PB, Malek GH, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. The Journal of Urology 2000;163:13‐20. - PubMed
Roehrborn 2002 {published data only}
-
- Roehrborn CG, McConnell JD, Saltzman B, Bergner D, Gray T, Narayan P, et al. Storage (irritative) and voiding (obstructive) symptoms predictors of benign prostatic hyperplasia progression and related outcomes. European Urology 2002;42:1‐6. - PubMed
Roehrborn 2004 {published data only}
-
- Roehrborn CG, Bruskewitz R, Nickel JC, McConnell JD, Saltzman B, Gittelman MC, et al. Sustained decrease in incidence of acute urinary retention and surgery with finasteride for 6 years in men with benign prostatic hyperplasia. The Journal of Urology 2004;171:1194‐8. - PubMed
Schäfer 1999 {published data only}
-
- Schäfer W, Tammela TLJ, Barrett DM, Abrams P, Hedlund H, Rollema HJ, et al. Continued improvement in pressure‐flow parameters in men receiving finasteride for 2 years. Urology 1999;54:278‐83. - PubMed
Siami 2007 {published data only}
-
- Siami P, Roehrborn CG, Barkin J, Damiao R, Wyczolkowski M, Duggan A, et al. Combination therapy with dutasteride and tamsulosin in men with moderate‐to‐severe benign prostatic hyperplasia and prostate enlargement: the CombAT (Combination of Avodart® and Tamsulosin) trial rationale and study design. Contemporary Clinical Trials 2007;28:770‐9. - PubMed
Stoner 1992a {published data only}
-
- Stoner E, Finasteride Study Group. The clinical effects of 5a‐reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Journal of Urology 1992;47:1298‐1302. - PubMed
Stoner 1994a {published data only}
-
- Stoner E, members of the Finasteride Study Group. Three‐year safety and efficacy data on the use of finasteride in the treatment of benign prostatic hyperplasia. Urology 1994;43(3):284‐94. - PubMed
Stoner 1994b {published data only}
-
- Stoner E, and the Finasteride Study Group. Maintenance of clinical efficacy with finasteride therapy for 24 months in patients with benign prostatic hyperplasia. Archives of Internal Medicine 1994;154:83‐8. - PubMed
Tammela 1995 {published data only}
-
- Tammela TLJ, Kontturi MJ. Long‐term effects of finasteride on invasive urodynamics and symptoms in the treatment of patients with bladder outflow obstruction due to benign prostatic hyperplasia. The Journal of Urology 1995;154:1466‐9. - PubMed
Tewari 1995 {published data only}
-
- Tewari A, Shinohara K, Narayan P. Transition zone volume and transition zone ratio: predictor or uroflow response to finasteride therapy in benign prostatic hyperplasia patients. Urology 1995;45(2):258‐65. - PubMed
Thompson 2003 {published data only}
-
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. The New England Journal of Medicine 2003;349(3):215‐24. - PubMed
Vaughan 2002 {published data only}
-
- Vaughan D, Imperato‐McGinley J, McConnell J, Matsumoto AM, Bracken B, Roy J, et al. Long‐term (7 to 8‐year) experience with finasteride in men with benign prostatic hyperplasia. Urology 2002;60:1040‐4. - PubMed
Additional references
Barry 1995
-
- Barry MJ, Williford WO, Chang Y, Machi M, Jones KM, Walker‐Corkery E, Lepor H. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients?. Journal of Urology 1995;154(5):1770‐5. - PubMed
Berry 1984
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. The Journal of Urology 1984;132:474‐9. - PubMed
Bonilla 1997
-
- Bonilla J, Stoner E, Grino P, Binkowitz B, Taylor A. Intra‐ and interobserver variability of MRI prostate volume measurements. The Prostate 1997;31:98‐102. - PubMed
Boyle 1996
-
- Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta‐analysis of randomized clinical trials. Urology 1996;48:398‐405. - PubMed
Byrnes 1997
-
- Byrnes CA, Liss CL, Tenover JL, Lippert MC, Gillenwater JY. Combined analysis of two multicenter studies of finasteride 5 mg in the treatment of symptomatic benign prostatic hyperplasia. Prostate Cancer and Prostatic Diseases 1997;1:26‐31. - PubMed
Campbell‐Walsh Urology 2007
-
- Roehrborn CG, McConnell JD. Chapter 86 ‐ Benign Prostatic Hyperplasia: Etiology, Pathophysiology, Epidemiology, and Natural History. In: Kavoussi LR, Novick AC, Partin AW, Peters CA editor(s). Campbell‐Walsh Urology. 9. Vol. 3, Philadelphia: Saunders Elsevier, 2007.
Cochrane Handbook 2008
-
- Higgins JPT, Green S, editors. Rationale for concern about bias. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008]. www.cochrane.org/resources/handbook/hbook.htm (accessed 17 February 2009).
Edwards 2002
García‐Perdomo 2015
-
- García‐Perdomo HA, Lopez HE, Tacklind J. 5‐alpha‐reductase inhibitors for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011928] - DOI
GRADE 2004
-
- GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1‐8.
Litwin, Saigal (editors) 2007
-
- Wei JT, Calhoun EA, Jacobsen SJ. Benign prostatic hyperplasia. In: Litwin MS, Saigal CS editor(s). Urologic Diseases of America. Washington, DC: US Government Publishing Office, 2007:45‐69.
Richardson 1995
-
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well‐built clinical question: a key to evidence‐based decisions. ACP Journal Club 1995;123(3):A12‐3. - PubMed
Rief 2002
-
- Rief W, Nestoriuc Y, Weiss S, Welzel E, Barsky AJ, Hofmann SG. Meta‐analysis of the placebo response in antidepressant trials. Journal of Affective Disorders 2009;118:1‐8. - PubMed
Roehrborn 1998
-
- Roehrborn CG. Meta‐analysis of randomized clinical trials of finasteride. Urology 1998;51(Suppl 4A):46‐9. - PubMed
Stoner 1992b
-
- Stoner E, Finasteride Study Group. The clinical effects of 5a‐reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Journal of Urology 1992;47:1298‐1302. - PubMed
Wessells 2003
-
- Wessells H, Roy J, Bannow J, Grayhack J, Matsumoto AM, Tenover L, et al. Incidence and severity of sexual adverse experiences in finasteride and placebo‐treated men with benign prostatic hyperplasia. Urology 2003;61:579‐84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

