Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices
- PMID: 20929283
- PMCID: PMC3043991
- DOI: 10.1089/ten.TEA.2010.0273
Direct comparisons of the morphology, migration, cell adhesions, and actin cytoskeleton of fibroblasts in four different three-dimensional extracellular matrices
Abstract
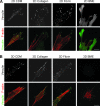
Interactions between cells and the extracellular matrix are at the core of tissue engineering and biology. However, most studies of these interactions have used traditional two-dimensional (2D) tissue culture, which is less physiological than three-dimensional (3D) tissue culture. In this study, we compared cell behavior in four types of commonly used extracellular matrix under 2D and 3D conditions. Specifically, we quantified parameters of cell adhesion and migration by human foreskin fibroblasts in cell-derived matrix or hydrogels of collagen type I, fibrin, or basement membrane extract (BME). Fibroblasts in 3D were more spindle shaped with fewer lateral protrusions and substantially reduced actin stress fibers than on 2D matrices; cells failed to spread in 3D BME. Cell-matrix adhesion structures were detected in all matrices. Although the shapes of these cell adhesions differed, the total area per cell occupied by cell-matrix adhesions in 2D and 3D was nearly identical. Fibroblasts migrated most rapidly in cell-derived 3D matrix and collagen and migrated minimally in BME, with highest migration directionality in cell-derived matrix. This identification of quantitative differences in cellular responses to different matrix composition and dimensionality should help guide the development of customized 3D tissue culture and matrix scaffolds for tissue engineering.
Figures






References
-
- Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. - PubMed
-
- Sun T. Jackson S. Haycock J.W. MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122:372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

