Patient-orientated longitudinal study of multiple sclerosis in south west England (The South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort
- PMID: 20929556
- PMCID: PMC2966453
- DOI: 10.1186/1471-2377-10-88
Patient-orientated longitudinal study of multiple sclerosis in south west England (The South West Impact of Multiple Sclerosis Project, SWIMS) 1: protocol and baseline characteristics of cohort
Abstract
Background: There is a need for greater understanding of the impact of multiple sclerosis (MS) from the perspective of individuals with the condition. The South West Impact of MS Project (SWIMS) has been designed to improve understanding of disease impact using a patient-centred approach. The purpose is to (1) develop improved measurement instruments for clinical trials, (2) evaluate longitudinal performance of a variety of patient-reported outcome measures, (3) develop prognostic predictors for use in individualising drug treatment for patients, particularly early on in the disease course.
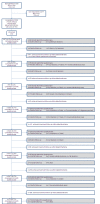
Methods: This is a patient-centred, prospective, longitudinal study of multiple sclerosis and clinically isolated syndrome (CIS) in south west England. The study area comprises two counties with a population of approximately 1.7 million and an estimated 1,800 cases of MS. Self-completion questionnaires are administered to participants every six months (for people with MS) or 12 months (CIS). Here we present descriptive statistics of the baseline data provided by 967 participants with MS.
Results: Seventy-five percent of those approached consented to participate. The male:female ratio was 1.00:3.01 (n = 967). Average (standard deviation) age at time of entry to SWIMS was 51.6 (11.5) years (n = 961) and median (interquartile range) time since first symptom was 13.3 (6.8 to 24.5) years (n = 934). Fatigue was the most commonly reported symptom, with 80% of participants experiencing fatigue at baseline. Although medication use for symptom control was common, there was little evidence of effectiveness, particularly for fatigue. Nineteen percent of participants were unable to classify their subtype of MS. When patient-reported subtype was compared to neurologist assessment for a sample of participants (n = 396), agreement in disease sub-type was achieved in 63% of cases. There were 836 relapses, reported by 931 participants, in the twelve months prior to baseline. Twenty-three percent of the relapsing-remitting group and 12% of the total sample were receiving disease-modifying therapy at baseline.
Conclusions: Demographics of this sample were similar to published data for the UK. Overall, the results broadly reflect clinical experience in confirming high symptom prevalence, with relatively little complete symptom relief. Participants often had difficulty in defining MS relapses and their own MS type.
Figures
References
-
- Giovannoni G, Thompson AJ, Miller DH, Thompson EJ. Fatigue is not associated with raised inflammatory markers in multiple sclerosis. Neurology. 2001;57(4):676–681. - PubMed
-
- Isaac C, Li DK, Genton M, Jardine C, Grochowski E, Palmer M, Kastrukoff LF, Oger J, Paty DW. Multiple sclerosis: a serial study using MRI in relapsing patients. Neurology. 1988;38(10):1511–1515. - PubMed
-
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. - PubMed
-
- Wolinsky JS, Narayana PA, O'Connor P, Coyle PK, Ford C, Johnson K, Miller A, Pardo L, Kadosh S, Ladkani D. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61(1):14–24. doi: 10.1002/ana.21079. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical