Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli
- PMID: 20929806
- PMCID: PMC3034138
- DOI: 10.1126/science.1191652
Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli
Abstract
Taxol (paclitaxel) is a potent anticancer drug first isolated from the Taxus brevifolia Pacific yew tree. Currently, cost-efficient production of Taxol and its analogs remains limited. Here, we report a multivariate-modular approach to metabolic-pathway engineering that succeeded in increasing titers of taxadiene--the first committed Taxol intermediate--approximately 1 gram per liter (~15,000-fold) in an engineered Escherichia coli strain. Our approach partitioned the taxadiene metabolic pathway into two modules: a native upstream methylerythritol-phosphate (MEP) pathway forming isopentenyl pyrophosphate and a heterologous downstream terpenoid-forming pathway. Systematic multivariate search identified conditions that optimally balance the two pathway modules so as to maximize the taxadiene production with minimal accumulation of indole, which is an inhibitory compound found here. We also engineered the next step in Taxol biosynthesis, a P450-mediated 5α-oxidation of taxadiene to taxadien-5α-ol. More broadly, the modular pathway engineering approach helped to unlock the potential of the MEP pathway for the engineered production of terpenoid natural products.
Figures
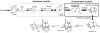
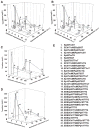


Comment in
-
Chemistry. A balancing act for Taxol precursor pathways in E. coli.Science. 2010 Oct 1;330(6000):44-5. doi: 10.1126/science.1195014. Science. 2010. PMID: 20929799 No abstract available.
References
-
- Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. J Am Chem Soc. 1971;93:2325. - PubMed
-
- Suffness M, Wall ME. In: Taxol: Science and Applications. Suffness M, editor. CRC; Boca Raton, FL: 1995. pp. 3–26.
-
- Nicolaou KC, et al. Nature. 1994;367:630. - PubMed
-
- Holton RA, et al. J Am Chem Soc. 1994;116:1597.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

