Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis
- PMID: 20929848
- PMCID: PMC3376907
- DOI: 10.1126/science.1192046
Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis
Abstract
The maintenance of a progenitor cell population as a reservoir of undifferentiated cells is required for organ development and regeneration. However, the mechanisms by which epithelial progenitor cells are maintained during organogenesis are poorly understood. We report that removal of the parasympathetic ganglion in mouse explant organ culture decreased the number and morphogenesis of keratin 5-positive epithelial progenitor cells. These effects were rescued with an acetylcholine analog. We demonstrate that acetylcholine signaling, via the muscarinic M1 receptor and epidermal growth factor receptor, increased epithelial morphogenesis and proliferation of the keratin 5-positive progenitor cells. Parasympathetic innervation maintained the epithelial progenitor cell population in an undifferentiated state, which was required for organogenesis. This mechanism for epithelial progenitor cell maintenance may be targeted for organ repair or regeneration.
Figures
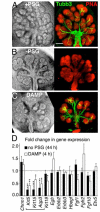
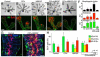

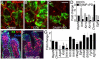
Comment in
-
Developmental biology. Branching takes nerve.Science. 2010 Sep 24;329(5999):1610-1. doi: 10.1126/science.1196016. Science. 2010. PMID: 20929836 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

