Role of PTH1R internalization in osteoblasts and bone mass using a phosphorylation-deficient knock-in mouse model
- PMID: 20929987
- PMCID: PMC3771320
- DOI: 10.1677/JOE-10-0227
Role of PTH1R internalization in osteoblasts and bone mass using a phosphorylation-deficient knock-in mouse model
Abstract
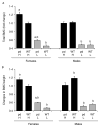
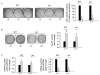
Phosphorylation, internalization, and desensitization of G protein-coupled receptors, such as the parathyroid hormone (PTH) and PTH-related peptide (PTHrP) receptor (PTH1R), are well characterized and known to regulate the cellular responsiveness in vitro. However, the role of PTH1R receptor phosphorylation in bone formation and osteoblast functions has not yet been elucidated. In previous studies, we demonstrated impaired internalization and sustained cAMP stimulation of a phosphorylation-deficient (pd) PTH1R in vitro, and exaggerated cAMP and calcemic responses to s.c. PTH infusion in pdPTH1R knock-in mouse model. In this study, we examined the impact of impaired PTH1R phosphorylation on the skeletal phenotype of mice maintained on normal, low, and high calcium diets. The low calcium diet moderately reduced (P<0.05) bone volume and trabecular number, and increased trabecular spacing in both wild-type (WT) and pd mice. The effects, however, seem to be less pronounced in the female pd compared to WT mice. In primary calvarial osteoblasts isolated from 2-week-old pd or WT mice, PTH and PTHrP decreased phosphorylated extracellular signal-regulated kinases 1/2 (pERK1/2), a member of mitogen-activated protein kinase, and cyclin D1, a G₁/S phase cyclin, in vitro. In contrast to WT osteoblasts, down-regulation of cyclin D1 was sustained for longer periods of time in osteoblasts isolated from the pd mice. Our results suggest that adaptive responses of intracellular signaling pathways in the pd mice may be important for maintaining bone homeostasis.
Conflict of interest statement
Figures

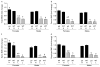
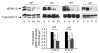

References
-
- Abou-Samra AB, Jueppner H, Potts JT, Jr, Segre GV. Inactivation of pertussis toxin-sensitive guanyl nucleotide-binding proteins increase parathyroid hormone receptors and reverse agonist-induced receptor down-regulation in ROS 17/2.8 cells. Endocrinology. 1989;125:2594–2599. doi: 10.1210/endo-125-5-2594. - DOI - PubMed
-
- Bounoutas GS, Tawfeek H, Frohlich LF, Chung UI, Abou-Samra AB. Impact of impaired receptor internalization on calcium homeostasis in knock-in mice expressing a phosphorylation-deficient parathyroid hormone (PTH)/PTH-related peptide receptor. Endocrinology. 2006;147:4674–4679. doi: 10.1210/en.2006-0301. - DOI - PubMed
-
- Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D'Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. Journal of Biological Chemistry. 2004;279:29121–29129. doi: 10.1074/jbc.M313000200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

