IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin
- PMID: 20930069
- PMCID: PMC3037756
- DOI: 10.1182/blood-2010-06-291245
IL-6 in human cytomegalovirus secretome promotes angiogenesis and survival of endothelial cells through the stimulation of survivin
Abstract
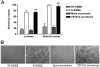
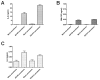
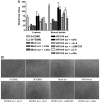
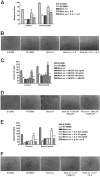
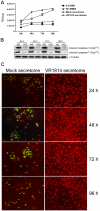
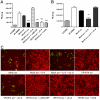
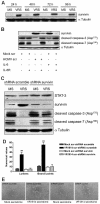
Human cytomegalovirus (HCMV) is linked to the acceleration of vascular diseases such as atherosclerosis and transplant vasculopathy. One of the hallmarks of these diseases is angiogenesis (AG) and neovessel formation. Endothelial cells (ECs) are an integral part of AG and are sites of HCMV persistence. AG requires multiple synchronous processes that include EC proliferation, migration, and vessel stabilization. Virus-free supernatant (secretome) from HCMV-infected ECs induces AG. To identify factor(s) involved in this process, we performed a human cytokine array. Several cytokines were significantly induced in the HCMV secretomes including interleukin-6 (IL-6), granulocyte macrophage colony-stimulating factor, and IL-8/CXCL8. Using in vitro AG assays, neutralization of IL-6 significantly reduced neovessel formation. Addition of the HCMV secretome to preformed vessels extended neovessel survival, but this effect was blocked by neutralization of IL-6. In these cells, IL-6 prevented apoptosis by blocking caspase-3 and -7 activation through the induction of survivin. Neutralization of IL-6 receptor on ECs abolished the ability of HCMV secretome to increase survivin expression and activated effector caspases. Moreover, survivin shRNA expression induced rapid regression of tubule capillary networks in ECs stimulated with HCMV secretome and activated effector caspases. These observations may explain how CMV accelerates vascular disease despite limited infection in tissues.
Figures
Similar articles
-
Human cytomegalovirus productively infects lymphatic endothelial cells and induces a secretome that promotes angiogenesis and lymphangiogenesis through interleukin-6 and granulocyte-macrophage colony-stimulating factor.J Gen Virol. 2011 Mar;92(Pt 3):650-60. doi: 10.1099/vir.0.025395-0. Epub 2010 Dec 1. J Gen Virol. 2011. PMID: 21123547
-
Human cytomegalovirus secretome contains factors that induce angiogenesis and wound healing.J Virol. 2008 Jul;82(13):6524-35. doi: 10.1128/JVI.00502-08. Epub 2008 Apr 30. J Virol. 2008. PMID: 18448536 Free PMC article.
-
Cytomegalovirus infection of vascular cells induces expression of pro-inflammatory adhesion molecules by paracrine action of secreted interleukin-1beta.Transplantation. 2000 Mar 27;69(6):1160-8. doi: 10.1097/00007890-200003270-00022. Transplantation. 2000. PMID: 10762222
-
Human cytomegalovirus infection and atherothrombosis.J Thromb Thrombolysis. 2012 Feb;33(2):160-72. doi: 10.1007/s11239-011-0662-x. J Thromb Thrombolysis. 2012. PMID: 22161772 Review.
-
Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing.Curr Top Microbiol Immunol. 2008;325:397-415. doi: 10.1007/978-3-540-77349-8_22. Curr Top Microbiol Immunol. 2008. PMID: 18637518 Free PMC article. Review.
Cited by
-
Genome-wide host responses against infectious laryngotracheitis virus vaccine infection in chicken embryo lung cells.BMC Genomics. 2012 Apr 24;13:143. doi: 10.1186/1471-2164-13-143. BMC Genomics. 2012. PMID: 22530940 Free PMC article.
-
Survivin expression promotes VEGF-induced tumor angiogenesis via PI3K/Akt enhanced β-catenin/Tcf-Lef dependent transcription.Mol Cancer. 2014 Sep 9;13:209. doi: 10.1186/1476-4598-13-209. Mol Cancer. 2014. PMID: 25204429 Free PMC article.
-
Cytomegalovirus infection enhances the immune response to influenza.Sci Transl Med. 2015 Apr 1;7(281):281ra43. doi: 10.1126/scitranslmed.aaa2293. Sci Transl Med. 2015. PMID: 25834109 Free PMC article.
-
An endothelial cell-specific requirement for the UL133-UL138 locus of human cytomegalovirus for efficient virus maturation.J Virol. 2013 Mar;87(6):3062-75. doi: 10.1128/JVI.02510-12. Epub 2013 Jan 2. J Virol. 2013. PMID: 23283945 Free PMC article.
-
Novel Strategies to Combat CMV-Related Cardiovascular Disease.Pathog Immun. 2020 Sep 20;5(1):240-274. doi: 10.20411/pai.v5i1.382. eCollection 2020. Pathog Immun. 2020. PMID: 33089035 Free PMC article. Review.
References
-
- Hosenpud JD, Shipley GD, Wagner CR. Cardiac allograft vasculopathy: current concepts, recent developments, and future directions. J Heart Lung Transplant. 1992;11(1 Pt 1):9–23. - PubMed
-
- Zhou YF, Leon MB, Waclawiw MW, et al. Association between prior cytomegalovirus infection and the risk of restenosis after coronary atherectomy. N Engl J Med. 1996;335(9):624–630. - PubMed
-
- Speir E, Modali R, Huang ES, et al. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265(5170):391–394. - PubMed
-
- Streblow DN, Soderberg-Naucler C, Vieira J, et al. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell. 1999;99(5):511–520. - PubMed
-
- Melnick JL, Adam E, DeBakey ME. The link between CMV and atherosclerosis. Infect Med. 1998;15:479–486.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

