Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility
- PMID: 20930086
- PMCID: PMC3052234
- DOI: 10.2215/CJN.04950610
Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility
Abstract
Background and objectives: Mesenchymal stromal cells (MSCs) abrogate alloimmune response in vitro, suggesting a novel cell-based approach in transplantation. Moving this concept toward clinical application in organ transplantation should be critically assessed.
Design, setting, participants & measurements: A safety and clinical feasibility study (ClinicalTrials.gov, NCT00752479) of autologous MSC infusion was conducted in two recipients of kidneys from living-related donors. Patients were given T cell-depleting induction therapy and maintenance immunosuppression with cyclosporine and mycophenolate mofetil. On day 7 posttransplant, MSCs were administered intravenously. Clinical and immunomonitoring of MSC-treated patients was performed up to day 360 postsurgery.
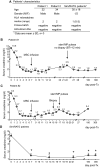
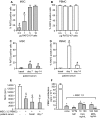
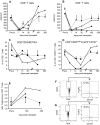
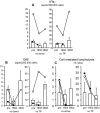
Results: Serum creatinine levels increased 7 to 14 days after cell infusion in both MSC-treated patients. A graft biopsy in patient 2 excluded acute graft rejection, but showed a focal inflammatory infiltrate, mostly granulocytes. In patient 1 protocol biopsy at 1-year posttransplant showed a normal graft. Both MSC-treated patients are in good health with stable graft function. A progressive increase of the percentage of CD4+CD25highFoxP3+CD127- Treg and a marked inhibition of memory CD45RO+RA-CD8+ T cell expansion were observed posttransplant. Patient T cells showed a profound reduction of CD8+ T cell activity.
Conclusions: Findings from this study in the two patients show that MSC infusion in kidney transplant recipients is feasible, allows enlargement of Treg in the peripheral blood, and controls memory CD8+ T cell function. Future clinical trials with MSCs to look with the greatest care for unwanted side effects is advised.
Figures

References
-
- Sayegh MH, Carpenter CB: Transplantation 50 years later–progress, challenges, and promises. N Engl J Med 351: 2761–2766, 2004 - PubMed
-
- Sayegh MH, Remuzzi G: Clinical update: Immunosuppression minimisation. Lancet 369: 1676–1678, 2007 - PubMed
-
- Millan MT, Shizuru JA, Hoffmann P, Dejbakhsh-Jones S, Scandling JD, Grumet FC, Tan JC, Salvatierra O, Hoppe RT, Strober S: Mixed chimerism and immunosuppressive drug withdrawal after HLA-mismatched kidney and hematopoietic progenitor transplantation. Transplantation 73: 1386–1391, 2002 - PubMed
-
- Shapiro R, BasGray E, Kahn A, Randhawa P, Basu A, Tan H, Murase N, Zeevi A, Girnita A, Metes D, Ness R, Bass DC, Demetris AJ, Fung JJ, Marcos A, Starzl TE: Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with Thymoglobulin or Campath. J Am Coll Surg 200: 505–515, quiz A559–A561, 2005 - PMC - PubMed
-
- Kirk AD, Mannon RB, Kleiner DE, Swanson JS, Kampen RL, Cendales LK, Elster EA, Wakefield T, Chamberlain C, Hoffmann SC, Hale DA: Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation 80: 1051–1059, 2005 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

