Manipulating the permeation of charged compounds through the MscL nanovalve
- PMID: 20930114
- PMCID: PMC3005423
- DOI: 10.1096/fj.10-170076
Manipulating the permeation of charged compounds through the MscL nanovalve
Abstract
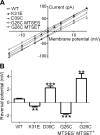
MscL is a bacterial mechanosensor that serves as a biological emergency release valve, releasing cytoplasmic solutes to the environment on osmotic downshock. Previous studies have recognized that this channel has properties that make it ideal for use as a triggered nanovalve for vesicular-based targeted drug-release devices. One can even change the modality of the sensor. Briefly, the introduction of charges into the MscL pore lumen gates the channel in the absence of membrane tension; thus, by inserting compounds that acquire a charge on exposure to an alternative stimulus, such as light or pH, into the pore of the channel, controllable nanoswitches that detect these alternative modalities have been engineered. However, a charge in the pore lumen could not only encourage actuation of the nanopore but also have a significant influence on the permeation of large charged compounds, which would thus have important implications for the efficiency of drug-release devices. In this study, we used in vivo and electrophysiological approaches to demonstrate that the introduction of a charge into pore lumen of MscL does indeed influence the permeation of charged molecules. These effects were more drastic for larger compounds and, surprisingly, were related to the orientation of the MscL channel in the membrane.
Figures
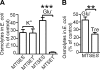

Similar articles
-
Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target.Microbiol Mol Biol Rev. 2020 Jan 15;84(1):e00055-19. doi: 10.1128/MMBR.00055-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31941768 Free PMC article. Review.
-
Breaking the hydrophobicity of the MscL pore: insights into a charge-induced gating mechanism.PLoS One. 2015 Mar 31;10(3):e0120196. doi: 10.1371/journal.pone.0120196. eCollection 2015. PLoS One. 2015. PMID: 25825909 Free PMC article.
-
Novel compounds that specifically bind and modulate MscL: insights into channel gating mechanisms.FASEB J. 2019 Mar;33(3):3180-3189. doi: 10.1096/fj.201801628R. Epub 2018 Oct 25. FASEB J. 2019. PMID: 30359098 Free PMC article.
-
Gating the bacterial mechanosensitive channel MscL invivo.Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5643-8. doi: 10.1073/pnas.082092599. Proc Natl Acad Sci U S A. 2002. PMID: 11960017 Free PMC article.
-
State-stabilizing Interactions in Bacterial Mechanosensitive Channel Gating and Adaptation.J Biol Chem. 2009 Jul 17;284(29):19153-7. doi: 10.1074/jbc.R109.009357. Epub 2009 Apr 21. J Biol Chem. 2009. PMID: 19383606 Free PMC article. Review.
Cited by
-
Controlled delivery of bioactive molecules into live cells using the bacterial mechanosensitive channel MscL.Nat Commun. 2012;3:990. doi: 10.1038/ncomms1999. Nat Commun. 2012. PMID: 22871809 Free PMC article.
-
Life with Bacterial Mechanosensitive Channels, from Discovery to Physiology to Pharmacological Target.Microbiol Mol Biol Rev. 2020 Jan 15;84(1):e00055-19. doi: 10.1128/MMBR.00055-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31941768 Free PMC article. Review.
-
Elucidating a role for the cytoplasmic domain in the Mycobacterium tuberculosis mechanosensitive channel of large conductance.Sci Rep. 2018 Oct 1;8(1):14566. doi: 10.1038/s41598-018-32536-6. Sci Rep. 2018. PMID: 30275500 Free PMC article.
-
Global redesign of a native β-barrel scaffold.Biochim Biophys Acta. 2016 Jan;1858(1):19-29. doi: 10.1016/j.bbamem.2015.10.006. Epub 2015 Oct 9. Biochim Biophys Acta. 2016. PMID: 26456555 Free PMC article.
-
The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves.J Bacteriol. 2012 Sep;194(18):4802-9. doi: 10.1128/JB.00576-12. Epub 2012 Jun 8. J Bacteriol. 2012. PMID: 22685280 Free PMC article. Review.
References
-
- Moe P. C., Blount P., Kung C. (1998) Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol. Microbiol. 28, 583–592 - PubMed
-
- Chang G., Spencer R. H., Lee A. T., Barclay M. T., Rees D. C. (1998) Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

