Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM)
- PMID: 20930118
- PMCID: PMC2972957
- DOI: 10.1073/pnas.1008924107
Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM)
Abstract
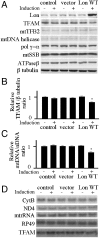
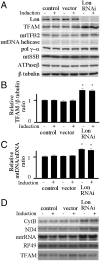
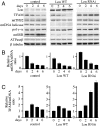
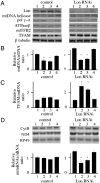
Lon is the major protease in the mitochondrial matrix in eukaryotes, and is well conserved among species. Although a role for Lon in mitochondrial biogenesis has been proposed, the mechanistic basis is unclear. Here, we demonstrate a role for Lon in mtDNA metabolism. An RNA interference (RNAi) construct was designed that reduces Lon to less than 10% of its normal level in Drosophila Schneider cells. RNAi knockdown of Lon results in increased abundance of mitochondrial transcription factor A (TFAM) and mtDNA copy number. In a corollary manner, overexpression of Lon reduces TFAM levels and mtDNA copy number. Notably, induction of mtDNA depletion in Lon knockdown cells does not result in degradation of TFAM, thereby causing a dramatic increase in the TFAMmtDNA ratio. The increased TFAMmtDNA ratio in turn causes inhibition of mitochondrial transcription. We conclude that Lon regulates mitochondrial transcription by stabilizing the mitochondrial TFAMmtDNA ratio via selective degradation of TFAM.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Koppen M, Langer T. Protein degradation within mitochondria: versatile activities of AAA proteases and other peptidases. Crit Rev Biochem Mol Biol. 2007;42:221–242. - PubMed
-
- Tsilibaris V, Maenhaut-Michel G, Van Melderen L. Biological roles of the Lon ATP-dependent protease. Res Microbiol. 2006;157:701–713. - PubMed
-
- Chandu D, Nandi D. Comparative genomics and functional roles of the ATP-dependent proteases Lon and Clp during cytosolic protein degradation. Res Microbiol. 2004;155:710–719. - PubMed
-
- Park SC, et al. Oligomeric structure of the ATP-dependent protease La (Lon) of Escherichia coli. Mol Cells. 2006;21:129–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

