Roles and interactions among protease-activated receptors and P2ry12 in hemostasis and thrombosis
- PMID: 20930120
- PMCID: PMC2972928
- DOI: 10.1073/pnas.1013309107
Roles and interactions among protease-activated receptors and P2ry12 in hemostasis and thrombosis
Abstract
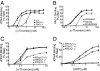
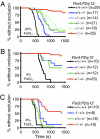
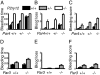
Toward understanding their redundancies and interactions in hemostasis and thrombosis, we examined the roles of thrombin receptors (protease-activated receptors, PARs) and the ADP receptor P2RY12 (purinergic receptor P2Y G protein-coupled 12) in human and mouse platelets ex vivo and in mouse models. Par3(-/-) and Par4(+/-) mouse platelets showed partially decreased responses to thrombin, resembling those in PAR1 antagonist-treated human platelets. P2ry12(+/-) mouse platelets showed partially decreased responses to ADP, resembling those in clopidogrel-treated human platelets. Par3(-/-) mice showed nearly complete protection against carotid artery thrombosis caused by low FeCl(3) injury. Par4(+/-) and P2ry12(+/-) mice showed partial protection. Increasing FeCl(3) injury abolished such protection; combining partial attenuation of thrombin and ADP signaling, as in Par3(-/-):P2ry12(+/-) mice, restored it. Par4(-/-) mice, which lack platelet thrombin responses, showed still better protection. Our data suggest that (i) the level of thrombin driving platelet activation and carotid thrombosis was low at low levels of arterial injury and increased along with the contribution of thrombin-independent pathways of platelet activation with increasing levels of injury; (ii) although P2ry12 acts downstream of PARs to amplify platelet responses to thrombin ex vivo, P2ry12 functioned in thrombin/PAR-independent pathways in our in vivo models; and (iii) P2ry12 signaling was more important than PAR signaling in hemostasis models; the converse was noted for arterial thrombosis models. These results make predictions being tested by ongoing human trials and suggest hypotheses for new antithrombotic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

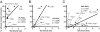
References
-
- Abrams CS, Brass LF. Platelet signal transduction. In: Coleman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice, 5th ed. Philadelphia: J. B. Lippincott,; 2006. pp. 617–629.
-
- Bhatt DL, et al. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. - PubMed
-
- TRA*CER Executive and Steering Committees. The Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRA*CER) trial: Study design and rationale. Am Heart J. 2009;158:327–334.e4. - PubMed
-
- Morrow DA, et al. Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: Design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2 degrees P)-TIMI 50 trial. Am Heart J. 2009;158:335–341.e3. - PubMed
-
- Davey MG, Lüscher EF. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967;216:857–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

