The various roles of ubiquitin in Wnt pathway regulation
- PMID: 20930545
- PMCID: PMC3047798
- DOI: 10.4161/cc.9.18.13204
The various roles of ubiquitin in Wnt pathway regulation
Abstract
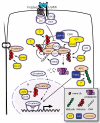
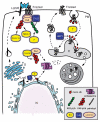
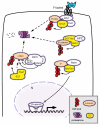
Wnt signaling mediates key developmental and homeostatic processes including stem cell maintenance, growth and cell fate specification, cell polarity and migration. Inappropriate activation of Wnt signaling is linked to a range of human disorders, most notably cancer and neurodegenerative diseases. In the Wnt/β-catenin cascade, signaling events converge on the regulation of ubiquitin-mediated degradation of the crucial transcriptional regulator β-catenin. The emerging mechanisms by which ubiquitin modification of proteins controls cellular pathways comprise both proteolytic and nonproteolytic functions. In nonproteolytic functions, ubiquitin acts as a signaling device in the control of protein activity, subcellular localization and complex formation. Here, we review and discuss recent developments that implicate ubiquitin-mediated mechanisms at multiple steps of Wnt pathway activation.
Figures
References
-
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:2–7. - PubMed
-
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38:439–446. - PubMed
-
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. - PubMed
-
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
