Integrin αIIbβ3: a novel effector of Gα13
- PMID: 20930548
- PMCID: PMC3038087
- DOI: 10.4161/cam.5.1.13559
Integrin αIIbβ3: a novel effector of Gα13
Abstract
Under physiological conditions, circulating platelets are discoid in shape. On these platelets, the fibrinogen receptor (integrin αIIbβ3) is in a low-affinity state, unable to bind soluble fibrinogen (Fg). Activation by agonists such as ADP and thrombin leads to a change in the conformation of the integrin αIIbβ3 through a process known as inside-out signaling. This enables the integrin to bind soluble Fg, which initiates a cascade of events referred to as outside-in signaling. Outside-in signaling control processes such as platelet spreading and clot retraction by regulating small G-proteins such as RhoA, Rac and cdc42.
Figures
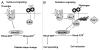
Comment on
-
G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin "outside-in" signaling.Science. 2010 Jan 15;327(5963):340-3. doi: 10.1126/science.1174779. Science. 2010. PMID: 20075254 Free PMC article.
References
-
- Siess W, Weber PC, Lapetina EG. Activation of phospholipase C is dissociated from arachidonate metabolism during platelet shape change induced by thrombin or platelet-activating factor. Epinephrine does not induce phospholipase C activation or platelet shape change. J Biol Chem. 1984;259:8286–8292. - PubMed
-
- Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–186. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
