Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition
- PMID: 20930847
- PMCID: PMC2966954
- DOI: 10.1038/embor.2010.151
Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition
Abstract
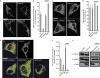
Trichoplein/mitostatin (TpMs) is a keratin-binding protein that partly colocalizes with mitochondria and is often downregulated in epithelial cancers, but its function remains unclear. In this study, we report that TpMs regulates the tethering between mitochondria and endoplasmic reticulum (ER) in a Mitofusin 2 (Mfn2)-dependent manner. Subcellular fractionation and immunostaining show that TpMs is present at the interface between mitochondria and ER. The expression of TpMs leads to mitochondrial fragmentation and loosens tethering with ER, whereas its silencing has opposite effects. Functionally, the reduced tethering by TpMs inhibits apoptosis by Ca(2+)-dependent stimuli that require ER-mitochondria juxtaposition. Biochemical and genetic evidence support a model in which TpMs requires Mfn2 to modulate mitochondrial shape and tethering. Thus, TpMs is a new regulator of mitochondria-ER juxtaposition.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


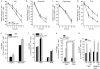
References
-
- Anesti V, Scorrano L (2006) The relationship between mitochondrial shape and function and the cytoskeleton. Biochim Biophys Acta 1757: 692–699 - PubMed
-
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 - PubMed
-
- Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L (2008) LETM1, deleted in Wolf Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum Mol Genet 17: 201–214 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

