Increasing the doses of both diuretics and angiotensin receptor blockers is beneficial in subjects with uncontrolled systolic hypertension
- PMID: 20931100
- PMCID: PMC2954540
- DOI: 10.1016/s0828-282x(10)70442-6
Increasing the doses of both diuretics and angiotensin receptor blockers is beneficial in subjects with uncontrolled systolic hypertension
Abstract
Background: Blood pressure (BP) control is frequently difficult to achieve in patients with predominantly elevated systolic BP. Consequently, these patients frequently require combination therapy including a thiazide diuretic such as hydrochlorothiazide (HCTZ) and an agent blocking the renin-angiotensin-aldosterone system. Current clinical practice usually limits the daily dose of HCTZ to 25 mg. This often leads to the necessity of using additional antihypertensive agents to control BP in a high proportion of patients.
Objectives: To compare the efficacy of two doses of losartan (LOS)⁄HCTZ combinations in patients with uncontrolled ambulatory systolic hypertension after six weeks of treatment with LOS 100 mg⁄HCTZ 25 mg (LOS100⁄HCTZ25).
Methods: Following a two- to four-week washout period, subjects with a mean clinic sitting systolic BP of 160 mmHg or higher and a mean ambulatory daytime systolic BP (MDSBP) of 135 mmHg or higher on LOS100⁄HCTZ25 (n=105; 33 women and 72 men) were randomly assigned to receive LOS 150 mg⁄HCTZ 25 mg (group 1; n=53) or LOS 150 mg⁄HCTZ 37.5 mg (LOS150⁄HCTZ37.5, group 2; n=52). The primary end point was the difference in MDSBP reductions.
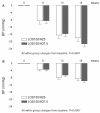
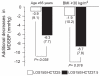
Results: At the end of the six-week treatment period, the respective additional decreases in MDSBP were 1.2 mmHg (P=0.335) on LOS 150 mg⁄HCTZ 25 mg and 5.6 mmHg (P<0.0001) on LOS150⁄HCTZ37.5 (difference of 4.4 mmHg; P=0.011). Daytime systolic ambulatory BP goal (lower than 130 mmHg) achievement tended to be higher (25% versus 17%; P=0.313) with LOS150⁄HCTZ37.5, while it was significantly higher (65% versus 43%; P=0.024) for mean daytime diastolic BP (lower than 80 mmHg). No deleterious metabolic changes were observed.
Conclusions: In patients with uncontrolled systolic ambulatory hypertension receiving LOS100⁄HCTZ25, increasing both HCTZ and LOS dosages simultaneously to LOS150⁄HCTZ37.5 may be an effective strategy that does not affect metabolic parameters.
HISTORIQUE :: Les patients qui ont une tension artérielle (TA) élevée à prédominance systolique éprouvent souvent de la difficulté à contrôler leur TA. Par conséquent, ces patients ont souvent besoin d’une polythérapie, incluant un diurétique thiazidique comme l’hydrochlorothiazide (HCTZ) et un agent bloquant le système rénine-angiotensine-aldostérone. D’ordinaire, la pratique clinique limite la dose quotidienne d’HCTZ à 25 mg, ce qui oblige à utiliser d’autres agents antihypertensifs chez une forte proportion de patients afin de contrôler la TA.
OBJECTIFS :: Comparer l’efficacité de deux doses de losartan (LOS) associée à l’HCTZ chez des patients dont l’hypertension systolique ambulatoire n’est pas contrôlée après six semaines de traitement de 100 mg au LOS et de 25 mg à l’HCTZ (100LOS/25HCTZ).
MÉTHODOLOGIE :: Après une période d’élimination de deux à quatre semaines, les sujets dont la TA systolique clinique moyenne en position assise était de 160 mmHg ou plus et la TA systolique ambulatoire moyenne de jour (TASMJ), de 135 mmHg ou plus et qui prenaient 100LOS/25HCTZ (n=105; 33 femmes et 72 hommes) ont été aléatoirement divisés pour recevoir 150 mg LOS/25 mg HCTZ (groupe 1; n=53) ou 150 mg LOS/37,5 mg HCTZ (150LOS/37,5HCTZ, groupe 2; n=52). Le paramètre ultime primaire était la différence de réductions de la TASMJ.
RÉSULTATS :: À la fin de la période de traitement de six semaines, les diminutions supplémentaires respectives de TASMJ étaient de 1,2 mmHg (P=0,335) chez les patients qui prenaient 150 mg LOS/25 mg HCTZ, et de 5,6 mmHg (P<0,0001) chez ceux qui prenaient 150LOS/37,5HCTZ (différence de 4,4 mmHg; P=0,011). L’objectif de TA systolique ambulatoire de jour (inférieure à 130 mmHg) tendait à être davantage atteint (25 % par rapport à 17 %; P=0,313) avec 150LOS/37,5HCTZ, mais l’était considérablement plus (65 % par rapport à 43 %; P=0,024) pour la TA diastolique moyenne de jour (inférieure à 80 mmHg). Aucune modification métabolique néfaste n’a été observée.
CONCLUSIONS :: Chez les patients dont l’hypertension systolique ambulatoire n’était pas contrôlée et qui recevaient 100LOS/25HCTZ, l’augmentation simultanée des doses d’HCTZ et de LOS à 150LOS/37,5HCTZ pourrait constituer une stratégie efficace qui n’influe pas sur les paramètres métaboliques.
Figures

Similar articles
-
Efficacy and safety of olmesartan medoxomil 40 mg/hydrochlorothiazide 12.5 mg combination therapy versus olmesartan medoxomil 40 mg monotherapy in patients with moderate to severe hypertension: a randomized, double-blind, parallel-group, multicentre, multinational, phase III study.Clin Drug Investig. 2010;30(9):581-97. doi: 10.2165/11536710-000000000-00000. Clin Drug Investig. 2010. PMID: 20593911 Clinical Trial.
-
Effects of force-titrated valsartan/hydrochlorothiazide versus amlodipine/hydrochlorothiazide on ambulatory blood pressure in patients with stage 2 hypertension: the EVALUATE study.Blood Press Monit. 2009 Jun;14(3):112-20. doi: 10.1097/MBP.0b013e32832a9da7. Blood Press Monit. 2009. PMID: 19384192 Clinical Trial.
-
Expedited blood pressure control with initial angiotensin II antagonist/diuretic therapy compared with stepped-care therapy in patients with ambulatory systolic hypertension.Can J Cardiol. 2007 Apr;23(5):377-82. doi: 10.1016/s0828-282x(07)70771-7. Can J Cardiol. 2007. PMID: 17440643 Free PMC article. Clinical Trial.
-
Combining angiotensin receptor blockers with chlorthalidone or hydrochlorothiazide - which is the better alternative? A meta-analysis.Syst Rev. 2020 Aug 24;9(1):195. doi: 10.1186/s13643-020-01457-9. Syst Rev. 2020. PMID: 32838806 Free PMC article. Review.
-
Efficacy of Zofenopril vs. Irbesartan in Combination with a Thiazide Diuretic in Hypertensive Patients with Multiple Risk Factors not Controlled by a Previous Monotherapy: A Review of the Double-Blind, Randomized "Z" Studies.Adv Ther. 2017 Apr;34(4):784-798. doi: 10.1007/s12325-017-0497-8. Epub 2017 Mar 4. Adv Ther. 2017. PMID: 28260186 Free PMC article. Review.
Cited by
-
Candesartan cilexetil 32 mg/hydrochlorothiazide 25 mg in unselected patients with high or very high cardiovascular risk: efficacy, safety, and metabolic impact.Clin Drug Investig. 2014 Apr;34(4):241-9. doi: 10.1007/s40261-014-0169-2. Clin Drug Investig. 2014. PMID: 24482018
-
Blood pressure-lowering efficacy of the fixed-dose combination of azilsartan medoxomil and chlorthalidone: a factorial study.J Clin Hypertens (Greenwich). 2012 May;14(5):284-92. doi: 10.1111/j.1751-7176.2012.00616.x. Epub 2012 Mar 6. J Clin Hypertens (Greenwich). 2012. PMID: 22533654 Free PMC article. Clinical Trial.
References
-
- McInnis NH, Fodor G, Moy Lum-Kwong M, Leenen FH. Antihypertensive medication use and blood pressure control: A community-based cross-sectional survey (ON-BP) Am J Hypertens. 2008;21:1210–5. - PubMed
-
- Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Eng J Med. 2001;345:479–86. - PubMed
-
- Mackay JH, Arcuri KE, Golberg AI, et al. Losartan and low dose hydrochlorothiazide in patients with essential hypertension. A double-blind, placebo-controlled trial of concomitant administration compared with individual components. Arch Intern Med. 1996;156:278–85. - PubMed
-
- Lacourcière Y, Poirier L. Antihypertensive effects of two fixed-dose combinations of losartan and hydrochlorothiazide versus hydrochlorothiazide monotherapy in subjects with ambulatory hypertension. Am J Hypertens. 2003;16:1036–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

