Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids
- PMID: 20931985
- PMCID: PMC3052696
- DOI: 10.1021/ol101981b
Efficient microwave-assisted synthesis of human islet amyloid polypeptide designed to facilitate the specific incorporation of labeled amino acids
Abstract
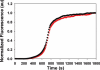
A cost-efficient, time-reducing solid-phase synthesis of the amyloidogenic, 37 residue islet amyloid polypeptide (IAPP) is developed using two pseudoprolines (highlighted blue in sequence) in combination with microwave technology. A yield twice that obtained with conventional syntheses is realized. The utility of this protocol is demonstrated by the synthesis of a (13)C(18)O-labeled Ser-20 IAPP variant, a prohibitively expensive and chemically challenging site to label via other protocols. TEM analysis shows the peptide forms normal amyloid (abstract image).
Figures

References
-
- Cooper G, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Proc. Natl. Acad. Sci. U.S.A. 1987;84:8628. - PMC - PubMed
- Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Proc. Natl. Acad. Sci. U.S.A. 1987;84:3881. - PMC - PubMed
- Kahn SE, D’Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ, Porte D. Diabetes. 1990;39:634. - PubMed
-
- Udayasankar J, Kodama K, Hull RL, Zraika S, Aston-Mourney K, Subramanian SL, Tong J, Faulenbach MV, Vidal J, Kahn SE. Diabetologia. 2009;52:143. - PMC - PubMed
- Westermark GT, Westermark P, Berne C, Korsgren O. N. Engl. J. Med. 2008;359:977. - PubMed
- Westermark P, Eizirik DL, Pipeleers DG, Hellerström C, Andersson A. Diabetologia. 1995;38:543. - PubMed
- Potter K, Abedini A, Marek P, Butterworth S, Driscoll M, Baker R, Nilsson M, Bertera S, Trucco M, Fraser PE, Raleigh DP, Verchere CB. Proc. Natl. Acad. Sci. 2010;107:4305. - PMC - PubMed
-
- Abedini A, Raleigh DP. Org. Lett. 2005;7:693. - PubMed
-
- Wöhr T, Wahl F, Nefzi A, Rohwedder B, Sato T, Sun X, Mutter M. J. Am. Chem. Soc. 1996;118:9218.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

