Highly stereoselective and scalable anti-aldol reactions using N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide: scope and origins of stereoselectivities
- PMID: 20932013
- PMCID: PMC2966945
- DOI: 10.1021/jo1015008
Highly stereoselective and scalable anti-aldol reactions using N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide: scope and origins of stereoselectivities
Abstract
A highly enantio- and diastereoselective anti-aldol process (up to >99% ee, >99:1 dr) catalyzed by a proline mimetic-N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide-has been developed. Catalyst loading as low as 2 mol % can be employed. Use of industry-friendly solvents for this transformation as well as neat reaction conditions have been demonstrated. The scope of this transformation on a range of aldehydes and ketones is explored. Density functional theory computations reveal that the origins of enhanced diastereoselectivity are due to the presence of nonclassical hydrogen bonds between the sulfonamide, the electrophile, and the catalyst enamine that favor the major anti-Re aldol TS in the Houk-List model.
Figures




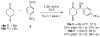
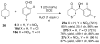
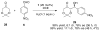
Similar articles
-
N-(p-dodecylphenylsulfonyl)-2-pyrrolidinecarboxamide: a practical proline mimetic for facilitating enantioselective aldol reactions.Org Lett. 2008 Oct 16;10(20):4649-52. doi: 10.1021/ol801941j. Epub 2008 Sep 23. Org Lett. 2008. PMID: 18808137 Free PMC article.
-
Enantioselective Mannich reactions with the practical proline mimetic N-(p-dodecylphenyl-sulfonyl)-2-pyrrolidinecarboxamide.J Org Chem. 2009 Mar 6;74(5):2246-9. doi: 10.1021/jo8027938. J Org Chem. 2009. PMID: 19193123
-
Density functional study of organocatalytic cross-aldol reactions between two aliphatic aldehydes: insight into their functional differentiation and origins of chemo- and stereoselectivities.J Phys Chem A. 2013 Apr 4;117(13):2862-72. doi: 10.1021/jp3126363. Epub 2013 Mar 21. J Phys Chem A. 2013. PMID: 23442005
-
Enantioselective aldol reactions catalyzed by chiral phosphine oxides.Chem Rec. 2013 Aug;13(4):362-70. doi: 10.1002/tcr.201300004. Epub 2013 Jul 4. Chem Rec. 2013. PMID: 23828817 Review.
-
Catalytic, enantioselective, vinylogous aldol reactions.Angew Chem Int Ed Engl. 2005 Jul 25;44(30):4682-98. doi: 10.1002/anie.200462338. Angew Chem Int Ed Engl. 2005. PMID: 15940727 Review.
Cited by
-
Proline Sulfonamide-Based Organocatalysis: Better Late than Never.Synlett. 2010 Sep 1;2010(19):2827-2838. doi: 10.1055/s-0030-1259020. Synlett. 2010. PMID: 21461132 Free PMC article.
-
Enantioselective approach to quinolizidines: total synthesis of cermizine D and formal syntheses of senepodine G and cermizine C.J Org Chem. 2013 May 17;78(10):4779-800. doi: 10.1021/jo400324t. Epub 2013 Apr 29. J Org Chem. 2013. PMID: 23627426 Free PMC article.
-
(Diisopinocampheyl)borane-mediated reductive aldol reactions of acrylate esters: enantioselective synthesis of anti-aldols.Org Lett. 2013 Aug 2;15(15):3922-5. doi: 10.1021/ol401679g. Epub 2013 Jul 25. Org Lett. 2013. PMID: 23885946 Free PMC article.
References
-
- Kane R. Ann Phys Chem. 1838;44:475. Ser. 2.
-
- Kekulé A. Ber Dtsch Chem Ges. 1869;2:365–368.
-
- Wurtz A. Bull Soc Chim Fr. 1872;17:436–442.
-
- Geary LM, Hultin PG. Tetrahedron: Asymm. 2009;20:131–173.
- Mahrwald R, editor. Modern Aldol Reactions. Wiley-VCH; 2004.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

