p27(Kip1) deficiency promotes prostate carcinogenesis but does not affect the efficacy of retinoids in suppressing the neoplastic process
- PMID: 20932324
- PMCID: PMC2958951
- DOI: 10.1186/1471-2407-10-541
p27(Kip1) deficiency promotes prostate carcinogenesis but does not affect the efficacy of retinoids in suppressing the neoplastic process
Abstract
Background: p27 is a cell cycle suppressor gene, whose protein is a negative regulator of cyclin/cdk complexes. p27 is also a potential target of retinoids in cancer prevention studies. In benign prostate hyperplasia (BPH), and in most carcinomas, p27(Kip1) is down-regulated, suggesting its potential resistance to retinoids. To test this hypothesis, we examined the efficacy of 9-cis retinoic acid (9cRA) to suppress prostate cell proliferation (PECP) and carcinogenesis in p27(Kip1) deficient mice.
Methods: p27(Kip1) deficient (-/-), heterozygous (+/-) and homozygous (+/+) mice were treated for 7 days with testosterone, 9cRA, or with both, and cell proliferation in dorsolateral prostate (DLP) was determined by BrdU labeling. Prostate carcinogenesis was induced by N-Methyl-N-Nitrosourea (MNU) and hormone stimulation.
Results: PECP in DLP of two-month-old mice of all genotypes was similar but significantly increased in old p27-/- mice only. Testosterone treatment increased PECP in all three p27 genotypes with the highest values in p27-/- mice. p27(Kip1) deficiency did not affect the response of PEC to 9cRA and to 9cRA+testosterone. The decrease of p27(Kip1) in p27+/- and p27-/- mice progressively increased the incidence and frequency of PIN and tumors. 9cRA suppressed PIN in all three p27 genotypes and this was associated with decreased PECP and increased cellular senescence.
Conclusions: This data indicates that p27(Kip1) deficiency promotes prostate cell proliferation and carcinogenesis but does not affect 9cRA's potential to suppress prostate carcinogenesis, suggesting that patients with PIN and carcinomas lacking or having a low level of p27(Kip1) expression may also benefit from clinical trials with retinoids.
Figures
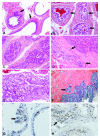
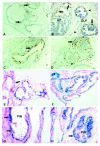
Similar articles
-
A critical role for p27kip1 gene dosage in a mouse model of prostate carcinogenesis.Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):17204-9. doi: 10.1073/pnas.0407693101. Epub 2004 Nov 29. Proc Natl Acad Sci U S A. 2004. PMID: 15569926 Free PMC article.
-
LncRNA GAS5 Inhibits Cellular Proliferation by Targeting P27Kip1.Mol Cancer Res. 2017 Jul;15(7):789-799. doi: 10.1158/1541-7786.MCR-16-0331. Epub 2017 Apr 10. Mol Cancer Res. 2017. PMID: 28396462
-
Reduced expression of the CDK inhibitor p27(KIP1) in rat two-stage bladder carcinogenesis and its association with expression profiles of p21(WAF1/Cip1) and p53.Carcinogenesis. 1999 Sep;20(9):1697-708. doi: 10.1093/carcin/20.9.1697. Carcinogenesis. 1999. PMID: 10469613
-
Susceptibility of p27 kip1 knockout mice to urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine may not simply be due to enhanced proliferation.Int J Cancer. 2008 Mar 15;122(6):1222-8. doi: 10.1002/ijc.23249. Int J Cancer. 2008. PMID: 18027869
-
p27(Kip1) signaling: Transcriptional and post-translational regulation.Int J Biochem Cell Biol. 2015 Nov;68:9-14. doi: 10.1016/j.biocel.2015.08.005. Epub 2015 Aug 14. Int J Biochem Cell Biol. 2015. PMID: 26279144 Review.
Cited by
-
ARv7 promotes the escape of prostate cancer cells from androgen deprivation therapy-induced senescence by mediating the SKP2/p27 axis.BMC Biol. 2025 Feb 28;23(1):66. doi: 10.1186/s12915-025-02172-4. BMC Biol. 2025. PMID: 40022149 Free PMC article.
References
-
- Alberti C. Hereditary/familial versus sporadic prostate cancer: few indisputable genetic differences and many similar clinicopathological features. Eur Rev Med Pharmacol Sci. 2010;14(1):31–41. - PubMed
-
- Roy S, Gu M, Ramasamy K, Singh RP, Agarwal C, Siriwardana S, Sclafani RA, Agarwal R. p21/Cip1 and p27/Kip1 Are essential molecular targets of inositol hexaphosphate for its antitumor efficacy against prostate cancer. Cancer Res. 2009;69(3):1166–1173. doi: 10.1158/0008-5472.CAN-08-3115. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

