Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions
- PMID: 20932325
- PMCID: PMC2964658
- DOI: 10.1186/1472-6793-10-19
Identification of differences in human and great ape phytanic acid metabolism that could influence gene expression profiles and physiological functions
Abstract
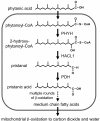
Background: It has been proposed that anatomical differences in human and great ape guts arose in response to species-specific diets and energy demands. To investigate functional genomic consequences of these differences, we compared their physiological levels of phytanic acid, a branched chain fatty acid that can be derived from the microbial degradation of chlorophyll in ruminant guts. Humans who accumulate large stores of phytanic acid commonly develop cerebellar ataxia, peripheral polyneuropathy, and retinitis pigmentosa in addition to other medical conditions. Furthermore, phytanic acid is an activator of the PPAR-alpha transcription factor that influences the expression of genes relevant to lipid metabolism.
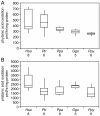
Results: Despite their trace dietary phytanic acid intake, all great ape species had elevated red blood cell (RBC) phytanic acid levels relative to humans on diverse diets. Unlike humans, chimpanzees showed sexual dimorphism in RBC phytanic acid levels, which were higher in males relative to females. Cultured skin fibroblasts from all species had a robust capacity to degrade phytanic acid. We provide indirect evidence that great apes, in contrast to humans, derive significant amounts of phytanic acid from the hindgut fermentation of plant materials. This would represent a novel reduction of metabolic activity in humans relative to the great apes.
Conclusion: We identified differences in the physiological levels of phytanic acid in humans and great apes and propose this is causally related to their gut anatomies and microbiomes. Phytanic acid levels could contribute to cross-species and sex-specific differences in human and great ape transcriptomes, especially those related to lipid metabolism. Based on the medical conditions caused by phytanic acid accumulation, we suggest that differences in phytanic acid metabolism could influence the functions of human and great ape nervous, cardiovascular, and skeletal systems.
Figures


Similar articles
-
Diverse captive non-human primates with phytanic acid-deficient diets rich in plant products have substantial phytanic acid levels in their red blood cells.Lipids Health Dis. 2013 Feb 4;12:10. doi: 10.1186/1476-511X-12-10. Lipids Health Dis. 2013. PMID: 23379307 Free PMC article.
-
Human and great ape red blood cells differ in plasmalogen levels and composition.Lipids Health Dis. 2011 Jun 17;10:101. doi: 10.1186/1476-511X-10-101. Lipids Health Dis. 2011. PMID: 21679470 Free PMC article.
-
Gravity and solidity in four great ape species (Gorilla gorilla, Pongo pygmaeus, Pan troglodytes, Pan paniscus): vertical and horizontal variations of the table task.J Comp Psychol. 2009 May;123(2):168-80. doi: 10.1037/a0013580. J Comp Psychol. 2009. PMID: 19450024
-
Comparative Pathology of Aging Great Apes: Bonobos, Chimpanzees, Gorillas, and Orangutans.Vet Pathol. 2016 Mar;53(2):250-76. doi: 10.1177/0300985815612154. Epub 2015 Dec 31. Vet Pathol. 2016. PMID: 26721908 Review.
-
An overview of nutritional factors in the aetiopathogenesis of myocardial fibrosis in great apes.Nutr Res Rev. 2025 Jun;38(1):37-52. doi: 10.1017/S0954422424000076. Epub 2024 Feb 12. Nutr Res Rev. 2025. PMID: 38343129 Review.
Cited by
-
Comparative RNA sequencing reveals substantial genetic variation in endangered primates.Genome Res. 2012 Apr;22(4):602-10. doi: 10.1101/gr.130468.111. Epub 2011 Dec 29. Genome Res. 2012. PMID: 22207615 Free PMC article.
-
A Generic Multivariate Framework for the Integration of Microbiome Longitudinal Studies With Other Data Types.Front Genet. 2019 Nov 7;10:963. doi: 10.3389/fgene.2019.00963. eCollection 2019. Front Genet. 2019. PMID: 31803221 Free PMC article.
-
Phytanic acid and the risk of non-Hodgkin lymphoma.Carcinogenesis. 2013 Jan;34(1):170-5. doi: 10.1093/carcin/bgs315. Epub 2012 Oct 5. Carcinogenesis. 2013. PMID: 23042099 Free PMC article.
-
The role of DNA insertions in phenotypic differentiation between humans and other primates.Genome Biol Evol. 2015 Jan 28;7(4):1168-78. doi: 10.1093/gbe/evv012. Genome Biol Evol. 2015. PMID: 25635043 Free PMC article.
-
Maternal malnutrition and anaemia in India: dysregulations leading to the 'thin-fat' phenotype in newborns.J Nutr Sci. 2021 Oct 11;10:e91. doi: 10.1017/jns.2021.83. eCollection 2021. J Nutr Sci. 2021. PMID: 34733503 Free PMC article. Review.
References
-
- Mitchell P. C. V. On the intestinal tract of mammals. Trans Zool Soc Lond. 1905;XVII:437–536.
-
- Milton K. The critical role played by animal source foods in human (Homo) evolution. J Nutr. 2003;133(11 Suppl 2):3886S–3892S. - PubMed
-
- Martin RD, Chivers DJ, Maclarnon AM, Hladik CM. In: Size and Scaling in Primate Biology. Jungers WJ, editor. Plenum: Plenum Press; 1985. Gastrointestinal allometry in primates and other mammals; pp. 61–139.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

