Multiple pathways of inhibition shape bipolar cell responses in the retina
- PMID: 20932357
- PMCID: PMC3222954
- DOI: 10.1017/S0952523810000209
Multiple pathways of inhibition shape bipolar cell responses in the retina
Abstract
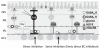
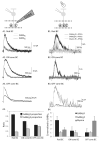
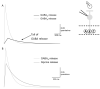
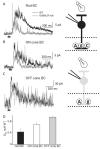
Bipolar cells (BCs) are critical relay neurons in the retina that are organized into parallel signaling pathways. The three main signaling pathways in the mammalian retina are the rod, ON cone, and OFF cone BCs. Rod BCs mediate incrementing dim light signals from rods, and ON cone and OFF cone BCs mediate incrementing and decrementing brighter light signals from cones, respectively. The outputs of BCs are shaped by inhibitory inputs from GABAergic and glycinergic amacrine cells in the inner plexiform layer, mediated by three distinct types of inhibitory receptors: GABA(A), GABA(C), and glycine receptors. The three main BC pathways receive distinct forms of inhibition from these three receptors that shape their light-evoked inhibitory signals. Rod BC inhibition is dominated by slow GABA(C) receptor inhibition, while OFF cone BCs are dominated by glycinergic inhibition. The inhibitory inputs to BCs are also shaped by serial inhibitory connections between GABAergic amacrine cells that limit the spatial profile of BC inhibition. We discuss our recent studies on how inhibitory inputs to BCs are shaped by receptor expression, receptor properties, and neurotransmitter release properties and how these affect the output of BCs.
Figures





Similar articles
-
Presynaptic inhibition differentially shapes transmission in distinct circuits in the mouse retina.J Physiol. 2007 Jul 15;582(Pt 2):569-82. doi: 10.1113/jphysiol.2007.131763. Epub 2007 Apr 26. J Physiol. 2007. PMID: 17463042 Free PMC article.
-
GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells.J Physiol. 2006 Apr 1;572(Pt 1):215-25. doi: 10.1113/jphysiol.2005.103648. Epub 2006 Jan 26. J Physiol. 2006. PMID: 16439422 Free PMC article.
-
Cannabinoid Signaling Selectively Modulates GABAergic Inhibitory Input to OFF Bipolar Cells in Rat Retina.Invest Ophthalmol Vis Sci. 2020 Mar 9;61(3):3. doi: 10.1167/iovs.61.3.3. Invest Ophthalmol Vis Sci. 2020. PMID: 32150246 Free PMC article.
-
Receptor targets of amacrine cells.Vis Neurosci. 2012 Jan;29(1):11-29. doi: 10.1017/S0952523812000028. Vis Neurosci. 2012. PMID: 22310370 Free PMC article. Review.
-
Retinal bipolar cells receive negative feedback input from GABAergic amacrine cells.Vis Neurosci. 1988;1(3):297-305. doi: 10.1017/s0952523800001954. Vis Neurosci. 1988. PMID: 2856476 Review.
Cited by
-
The temporal structure of the inner retina at a single glance.Sci Rep. 2020 Mar 10;10(1):4399. doi: 10.1038/s41598-020-60214-z. Sci Rep. 2020. PMID: 32157103 Free PMC article.
-
Possible roles of glutamate transporter EAAT5 in mouse cone depolarizing bipolar cell light responses.Vision Res. 2014 Oct;103:63-74. doi: 10.1016/j.visres.2014.06.005. Epub 2014 Jun 24. Vision Res. 2014. PMID: 24972005 Free PMC article.
-
Functional Circuitry of the Retina.Annu Rev Vis Sci. 2015 Nov 24;1:263-289. doi: 10.1146/annurev-vision-082114-035334. Annu Rev Vis Sci. 2015. PMID: 28532365 Free PMC article.
-
Synaptic inhibition tunes contrast computation in the retina.Vis Neurosci. 2019 Jan;36:E006. doi: 10.1017/S095252381900004X. Vis Neurosci. 2019. PMID: 31199207 Free PMC article.
-
The neuronal organization of the retina.Neuron. 2012 Oct 18;76(2):266-80. doi: 10.1016/j.neuron.2012.10.002. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083731 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

