How does public policy impact cervical screening and vaccination strategies?
- PMID: 20932433
- PMCID: PMC4443709
- DOI: 10.1016/j.ygyno.2010.08.021
How does public policy impact cervical screening and vaccination strategies?
Abstract
Objectives: To examine the current approaches to cervical screening and points to consider for improving HPV vaccination acceptance and uptake in the US.
Methods: An expert forum was conducted September 12-13, 2008, by the Society of Gynecologic Oncologists including 56 experts in cervical cancer and titled "Future Strategies of Cervical Cancer Prevention: What Do We Need to Do Now to Prepare?".
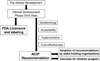
Results: Cervical cancer prevention has primarily relied on screening paradigms but vaccination against human papillomavirus (HPV), the cause of the disease, is a primary preventative measure that has been recommended by all cervical cancer screening stakeholders. Guidelines for vaccination are developed by national advisory groups, but successful implementation requires a supportive infrastructure and the cooperation of providers, clinicians, and patients. HPV vaccination has been available in the United States (US) since 2006 and screening practices have been updated to also include HPV genotyping. However, many clinicians fail to adhere to the guidelines for HPV testing (and HPV co-testing) as part of cervical cancer screening, and vaccination coverage has been poor among females aged 11 and 12, the group for which vaccination is recommended by all organizations.
Conclusions: The data reviewed and presented in this session of the "Future Strategies of Cervical Cancer Prevention. What Do We Need to do Now to Prepare?". The Forum suggests that the policies influencing HPV vaccination and screening need to be reassessed at multiple levels in order to achieve more effective implementation and regular use.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
T. Herzog- Honoraria for educational programs from GSK and Merck M. Einstein- Dr. Einstein has advised or participated in educational speaking activities, but does not receive an honorarium from any companies. In specific cases, Montefiore Medical Center has received payment for time spent for these activities from Merck, GSK, Roche, Hologic, Advaxis, Aura Biosciences, and PDS Biotechnologies. Also, Montefiore has received grant funding for research related costs of clinical trials that Dr. Einstein has been the Montefiore PI from Merck, GSK, Roche, and Hologic. W. Huh- Consultant: Merck, GSK, Roche, Hologic, and Helix BioPharma; Speaker: Merck, GSK; Research Support: Merck, GSK and Roche.
Figures


References
-
- FDA Approves Expanded Use of HPV Test. US Food and Drug Administration. [Accessed April 5, 2007]; http://www.fda.gov/bbs/topics/NEWS/2003/NEW00890.html.
-
- Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002 Nov-Dec;52(6):342–362. - PubMed
-
- Saraiya M, Berkowitz Z, Yabroff R, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and papanicolaou testing vs Papanicolaou testing alone, what screening intervals are physicians recommending? Arch Intern Med. 2010;170(11):977–986. - PubMed
-
- Yabroff KR, Saraiya M, Meissner HI, Haggstrom DA, Wideroff L, Yuan G, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann Intern Med. 2009 Nov 3;151(9):602–611. - PubMed
-
- Moriarty AT, Schwartz MR, Eversole G, et al. Human papillomavirus testing and reporting rates: practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Gynecologic Cytology in 2006. Arch Pathol Lab Med. 2008 Aug;132(8):1290–1294. - PubMed

