Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals
- PMID: 20932495
- PMCID: PMC3100475
- DOI: 10.1016/j.lfs.2010.09.012
Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals
Abstract
Multidrug regimens and corresponding drug interactions cause many adverse reactions and treatment failures. Drug efflux transporters: P-gp, MRP, BCRP in conjunction with metabolizing enzymes (CYPs) are major factors in such interactions. Most effective combination antiretrovirals (ARV) therapy includes a PI or a NNRTI or two NRTI. Coadministration of such ARV may induce efflux transporters and/or CYP3A4 resulting in sub-therapeutic blood levels and therapeutic failure due to reduced absorption and/or increased metabolism. A similar prognosis is true for ARV-compounds and drugs of abuse combinations. Morphine and nicotine enhance CYP3A4 and MDR1 expression in vitro. A 2.5 fold rise of cortisol metabolite was evident in smokers relative to nonsmokers. Altered functions of efflux transporters and CYPs in response to ARV and drugs of abuse may result in altered drug absorption and metabolism. Appropriate in vitro models can be employed to predict such interactions. Influence of genetic polymorphism, SNP and inter-individual variation in drug response has been discussed. Complexity underlying the relationship between efflux transporters and CYP makes it difficult to predict the outcome of HAART as such, particularly when HIV patients taking drugs of abuse do not adhere to HAART regimens. HIV(+) pregnant women on HAART medications, indulging in drugs of abuse, may develop higher viral load due to such interactions and lead to increase in mother to child transmission of HIV. A multidisciplinary approach with clear understanding of mechanism of interactions may allow proper selection of regimens so that desired therapeutic outcome of HAART can be reached without any side effects.
Published by Elsevier Inc.
Figures

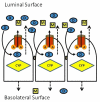
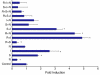






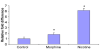




References
-
- Altice FL, Friedland GH, Cooney EL. Nevirapine induced opiate withdrawal among injection drug users with HIV infection receiving methadone. AIDS. 1999;13(8):957–62. - PubMed
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–98. - PubMed
-
- Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, part I. Psychosomatics. 2003a;44(2):167–71. - PubMed
-
- Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, Part II. Psychosomatics. 2003b;44(6):515–20. - PubMed
-
- Baker JR, Best AM, Pade PA, McCance-Katz EF. Effect of buprenorphine and antiretroviral agents on the QT interval in opioid-dependent patients. Ann Pharmacother. 2006;40(3):392–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

