The functional role of cross-frequency coupling
- PMID: 20932795
- PMCID: PMC3359652
- DOI: 10.1016/j.tics.2010.09.001
The functional role of cross-frequency coupling
Abstract
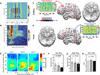
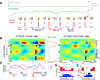
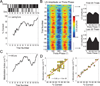
Recent studies suggest that cross-frequency coupling (CFC) might play a functional role in neuronal computation, communication and learning. In particular, the strength of phase-amplitude CFC differs across brain areas in a task-relevant manner, changes quickly in response to sensory, motor and cognitive events, and correlates with performance in learning tasks. Importantly, whereas high-frequency brain activity reflects local domains of cortical processing, low-frequency brain rhythms are dynamically entrained across distributed brain regions by both external sensory input and internal cognitive events. CFC might thus serve as a mechanism to transfer information from large-scale brain networks operating at behavioral timescales to the fast, local cortical processing required for effective computation and synaptic modification, thus integrating functional systems across multiple spatiotemporal scales.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press; 2006.
-
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2001;2:229–239. - PubMed
-
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001;2:704–716. - PubMed
-
- Lakatos P, et al. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J. Neurophysiol. 2005;94:1904–1911. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

