New aspects of melanocortin signaling: a role for PRCP in α-MSH degradation
- PMID: 20932857
- PMCID: PMC4766861
- DOI: 10.1016/j.yfrne.2010.09.001
New aspects of melanocortin signaling: a role for PRCP in α-MSH degradation
Abstract
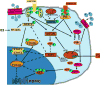
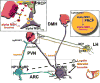
The role of the central melanocortin system in the regulation of energy metabolism has received much attention during the past decade since gene mutations of key components in melanocortin signaling cause monogenic forms of obesity in animals and humans. In the arcuate nucleus of the hypothalamus the prohormone proopiomelanocortin (POMC) is posttranslationally cleaved to produce α-melanocyte stimulating hormone (α-MSH), a peptide with anorexigenic effects upon activation of the melanocortin receptors (MCRs). α-MSH undergoes extensive post-translational processing and its in vivo activity is short lived due to rapid degradation. The enzymatic process that controls α-MSH inactivation is incompletely understood. Recent evidence suggests that prolyl carboxypeptidase (PRCP) is an enzyme responsible for α-MSH degradation. As for many key melanocortin peptides, gene mutation of PRCP causes a change in the metabolic phenotype of rodents. This review summarizes the current knowledge on the melanocortin system with particular focus on PRCP, a newly discovered component of the melanocortin system.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

References
-
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–8. - PubMed
-
- Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, Foretz M, Thorens B. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–95. - PubMed
-
- Bagnasco M, Dube MG, Kalra PS, Kalra SP. Evidence for the existence of distinct central appetite, energy expenditure, and ghrelin stimulation pathways as revealed by hypothalamic site-specific leptin gene therapy. Endocrinology. 2002;143:4409–4421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

