Calcium-mediated mechanisms of cystic expansion
- PMID: 20932898
- PMCID: PMC3043160
- DOI: 10.1016/j.bbadis.2010.09.016
Calcium-mediated mechanisms of cystic expansion
Abstract
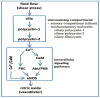
In this review, we will discuss several well-accepted signaling pathways toward calcium-mediated mechanisms of cystic expansion. The second messenger calcium ion has contributed to a vast diversity of signal transduction pathways. We will dissect calcium signaling as a possible mechanism that contributes to renal cyst formation. Because cytosolic calcium also regulates an array of signaling pathways, we will first discuss cilia-induced calcium fluxes, followed by Wnt signaling that has attributed to much-discussed planar cell polarity. We will then look at the relationship between cytosolic calcium and cAMP as one of the most important aspects of cyst progression. The signaling of cAMP on MAPK and mTOR will also be discussed. We infer that while cilia-induced calcium fluxes may be the initial signaling messenger for various cellular pathways, no single signaling mediator or pathway is implicated exclusively in the progression of the cystic expansion. This article is part of a Special Issue entitled: Polycystic Kidney Disease.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium, Cell. 1995;81:289–298. - PubMed
-
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein, Science. Vol. 272. New York, N.Y: 1996. pp. 1339–1342. - PubMed
-
- Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nature genetics. 2002;30:259–269. - PubMed
-
- Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. The Journal of biological chemistry. 1999;274:28557–28565. - PubMed
-
- Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernandez-Fernandez JM, Harris P, Frischauf AM, Brown DA, Zhou J. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. The Journal of biological chemistry. 2002;277:11276–11283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

