Modification of ubiquitin-C-terminal hydrolase-L1 by cyclopentenone prostaglandins exacerbates hypoxic injury
- PMID: 20933087
- PMCID: PMC3014436
- DOI: 10.1016/j.nbd.2010.09.020
Modification of ubiquitin-C-terminal hydrolase-L1 by cyclopentenone prostaglandins exacerbates hypoxic injury
Abstract
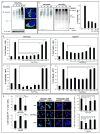
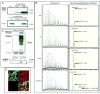
Cyclopentenone prostaglandins (CyPGs), such as 15-deoxy-Δ(12,14) -prostaglandin J(2) (15d-PGJ(2)), are active prostaglandin metabolites exerting a variety of biological effects that may be important in the pathogenesis of neurological diseases. Ubiquitin-C-terminal hydrolase L1 (UCH-L1) is a brain specific deubiquitinating enzyme whose aberrant function has been linked to neurodegenerative disorders. We report that [15d-PGJ(2)] detected by quadrapole mass spectrometry (MS) increases in rat brain after temporary focal ischemia, and that treatment with 15d-PGJ(2) induces accumulation of ubiquitinated proteins and exacerbates cell death in normoxic and hypoxic primary neurons. 15d-PGJ(2) covalently modifies UCH-L1 and inhibits its hydrolase activity. Pharmacologic inhibition of UCH-L1 exacerbates hypoxic neuronal death while transduction with a TAT-UCH-L1 fusion protein protects neurons from hypoxia. These studies indicate that UCH-L1 function is important in hypoxic neuronal death and that excessive production of CyPGs after stroke may exacerbate ischemic injury by modification and inhibition of UCH-L1.
Published by Elsevier Inc.
Conflict of interest statement
Figures




Similar articles
-
The point mutation UCH-L1 C152A protects primary neurons against cyclopentenone prostaglandin-induced cytotoxicity: implications for post-ischemic neuronal injury.Cell Death Dis. 2015 Nov 5;6(11):e1966. doi: 10.1038/cddis.2015.323. Cell Death Dis. 2015. PMID: 26539913 Free PMC article.
-
Increased generation of cyclopentenone prostaglandins after brain ischemia and their role in aggregation of ubiquitinated proteins in neurons.Neurotox Res. 2013 Aug;24(2):191-204. doi: 10.1007/s12640-013-9377-4. Epub 2013 Jan 25. Neurotox Res. 2013. PMID: 23355003 Free PMC article.
-
Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1.Proc Natl Acad Sci U S A. 2010 Apr 13;107(15):6835-40. doi: 10.1073/pnas.1002295107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231490 Free PMC article.
-
Ubiquitin C-terminal hydrolase L1 (UCH-L1): structure, distribution and roles in brain function and dysfunction.Biochem J. 2016 Aug 15;473(16):2453-62. doi: 10.1042/BCJ20160082. Biochem J. 2016. PMID: 27515257 Free PMC article. Review.
-
15-Deoxy-Delta(12,14)-prostaglandin J2: an electrophilic trigger of cellular responses.Chem Res Toxicol. 2008 Jan;21(1):138-44. doi: 10.1021/tx700177j. Epub 2007 Dec 4. Chem Res Toxicol. 2008. PMID: 18052108 Review.
Cited by
-
Mitochondrial and calcium perturbations in rat CNS neurons induce calpain-cleavage of Parkin: Phosphatase inhibition stabilizes pSer65Parkin reducing its calpain-cleavage.Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1436-1450. doi: 10.1016/j.bbadis.2019.02.016. Epub 2019 Feb 21. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30796971 Free PMC article.
-
Protein Modifications with Ubiquitin as Response to Cerebral Ischemia-Reperfusion Injury.Transl Stroke Res. 2018 Apr;9(2):157-173. doi: 10.1007/s12975-017-0567-x. Epub 2017 Aug 25. Transl Stroke Res. 2018. PMID: 28842824 Review.
-
Abolishing UCHL1's hydrolase activity exacerbates TBI-induced axonal injury and neuronal death in mice.Exp Neurol. 2021 Feb;336:113524. doi: 10.1016/j.expneurol.2020.113524. Epub 2020 Nov 4. Exp Neurol. 2021. PMID: 33159930 Free PMC article.
-
Increased cytochrome c in rat cerebrospinal fluid after cardiac arrest and its effects on hypoxic neuronal survival.Resuscitation. 2012 Dec;83(12):1491-6. doi: 10.1016/j.resuscitation.2012.04.009. Epub 2012 May 1. Resuscitation. 2012. PMID: 22554683 Free PMC article.
-
The requirement of ubiquitin C-terminal hydrolase L1 in mouse ovarian development and fertility†.Biol Reprod. 2022 Aug 9;107(2):500-513. doi: 10.1093/biolre/ioac086. Biol Reprod. 2022. PMID: 35512140 Free PMC article.
References
-
- Aldini G, Carini M, Vistoli G, Shibata T, Kusano Y, Gamberoni L, Dalle-Donne I, Milzani A, Uchida K. Identification of actin as a 15-deoxy-Delta12,14-prostaglandin J2 target in neuroblastoma cells: mass spectrometric, computational, and functional approaches to investigate the effect on cytoskeletal derangement. Biochemistry. 2007;46:2707–18. - PubMed
-
- Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L. Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson's and Alzheimer's diseases. J Biol Chem. 2004;279:13256–64. - PubMed
-
- Colgan L, Liu H, Huang SY, Liu YJ. Dileucine motif is sufficient for internalization and synaptic vesicle targeting of vesicular acetylcholine transporter. Traffic. 2007;8:512–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

