The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels
- PMID: 20933268
- PMCID: PMC3004019
- DOI: 10.1016/j.biomaterials.2010.08.103
The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels
Abstract
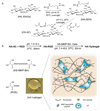
Synthetic hydrogel scaffolds that can be used as culture systems that mimic the natural stem cell niche are of increased importance for stem cell biology and regenerative medicine. These artificial niches can be utilized to control the stem cell fate and will have potential applications for expanding/differentiating stem cells in vitro, delivering stem cells in vivo, as well as making tissue constructs. In this study, we synthesized hyaluronic acid (HA) hydrogels that could be degraded through a combination of cell-released enzymes and used them to culture mouse mesenchymal stem cells (mMSC). To form the hydrogels, HA was modified to contain acrylate groups and crosslinked through Michael addition chemistry using non-degradable, plasmin degradable or matrix metalloproteinase (MMP) degradable crosslinkers. Using this hydrogel we found that mMSC proliferation occurred in the absence of cell spreading, that mMSCs could only spread when both RGD and MMP degradation sites were present in the hydrogel and that mMSCs in hydrogels with high density of RGD (1000 μm) spread and migrated faster and more extensively than in hydrogels with low density of RGD (100 μm).
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures





References
-
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. - PubMed
-
- Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453(7193):322–329. - PubMed
-
- Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. Neurosci . Res . 2009;87(10):2183–2200. - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. - PubMed
-
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat . Biotechnol . 2000;18(4):399–404. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

