Stem cell homing in musculoskeletal injury
- PMID: 20933277
- PMCID: PMC2991369
- DOI: 10.1016/j.biomaterials.2010.08.101
Stem cell homing in musculoskeletal injury
Abstract
The regenerative potential of injured adult tissue suggests the physiological existence of cells capable of participating in the reparative process. Recent studies indicate that stem-like cells residing in tissues contribute to tissue repair and are replenished by precursor bone marrow-derived cells. Mesenchymal stromal cells (MSC) are among the candidates for reparative cells. These cells can potentially be mobilized into the circulation in response to injury signals and exert their reparative effects at the site of injury. Current therapies for musculoskeletal injuries pose unavoidable risks which can impede full recovery. Trafficking of MSC to the injury site and their subsequent participation in the regenerative process is thought to be a natural healing response that can be imitated or augmented by enhancing the endogenous MSC pool with exogenously administered MSC. Therefore, a promising alternative to the existing strategies employed in the treatment of musculoskeletal injuries is to reinforce the inherent reparative capacity of the body by delivering MSC harvested from the patient's own tissues to the site of injury. The aim of this review is to inform the reader of studies that have evaluated the intrinsic homing and regenerative abilities of MSC, with particular emphasis on the repair of musculoskeletal injuries. Research that supports the direct use of MSC (without in vitro differentiation into tissue-specific cells) will also be reported. Based on accruing evidence that the natural healing mechanism involves the recruitment of MSC and their subsequent reparative actions at the site of injury, as well as documented therapeutic response after the exogenous administration of MSC, the feasibility of the emerging strategy of instant stem-cell therapy will be proposed.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures

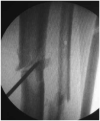
References
-
- Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther. 2008;8(3):255–68. - PubMed
-
- Roberts SJ, Howard D, Buttery LD, Shakesheff KM. Clinical applications of musculoskeletal tissue engineering. Br Med Bull. 2008;86:7–22. - PubMed
-
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–99. - PubMed
-
- Liu ZJ, Zhuge Y, Velazquez OC. Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem. 2009;106(6):984–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

