Cse1l is a negative regulator of CFTR-dependent fluid secretion
- PMID: 20933420
- PMCID: PMC2963654
- DOI: 10.1016/j.cub.2010.09.012
Cse1l is a negative regulator of CFTR-dependent fluid secretion
Erratum in
- Curr Biol. 2010 Dec 7;20(23):2157
Abstract
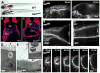
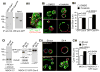
Transport of chloride through the cystic fibrosis transmembrane conductance regulator (CFTR) channel is a key step in regulating fluid secretion in vertebrates [1, 2]. Loss of CFTR function leads to cystic fibrosis [1, 3, 4], a disease that affects the lungs, pancreas, liver, intestine, and vas deferens. Conversely, uncontrolled activation of the channel leads to increased fluid secretion and plays a major role in several diseases and conditions including cholera [5, 6] and other secretory diarrheas [7] as well as polycystic kidney disease [8-10]. Understanding how CFTR activity is regulated in vivo has been limited by the lack of a genetic model. Here, we used a forward genetic approach in zebrafish to uncover CFTR regulators. We report the identification, isolation, and characterization of a mutation in the zebrafish cse1l gene that leads to the sudden and dramatic expansion of the gut tube. We show that this phenotype results from a rapid accumulation of fluid due to the uncontrolled activation of the CFTR channel. Analyses in zebrafish larvae and mammalian cells indicate that Cse1l is a negative regulator of CFTR-dependent fluid secretion. This work demonstrates the importance of fluid homeostasis in development and establishes the zebrafish as a much-needed model system to study CFTR regulation in vivo.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem. 2008;77:701–726. - PubMed
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. - PubMed
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Gabriel SE, Brigman KN, Koller BH, Boucher RC, Stutts MJ. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK083330/DK/NIDDK NIH HHS/United States
- P01AI53194/AI/NIAID NIH HHS/United States
- P01 AI053194/AI/NIAID NIH HHS/United States
- DK075032/DK/NIDDK NIH HHS/United States
- 1DP2OD006486/OD/NIH HHS/United States
- DK074398/DK/NIDDK NIH HHS/United States
- P30 DK065988/DK/NIDDK NIH HHS/United States
- R01 DK067153/DK/NIDDK NIH HHS/United States
- R01 DK060322/DK/NIDDK NIH HHS/United States
- DK083330/DK/NIDDK NIH HHS/United States
- DP2 OD006486/OD/NIH HHS/United States
- R01 DK075032/DK/NIDDK NIH HHS/United States
- DK60322/DK/NIDDK NIH HHS/United States
- R01 DK074398/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

