Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring
- PMID: 20934214
- PMCID: PMC3042747
- DOI: 10.1016/j.biomaterials.2010.09.019
Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring
Abstract
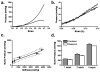
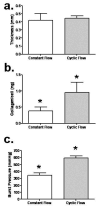
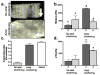
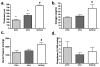
Tissue-engineered arteries based on entrapment of human dermal fibroblasts in fibrin gel yield completely biological vascular grafts that possess circumferential alignment characteristic of native arteries and essential to their mechanical properties. A bioreactor was developed to condition six grafts in the same culture medium while being subjected to similar cyclic distension and transmural flow resulting from pulsed flow distributed among the graft lumens via a manifold. The lumenal pressure and circumferential stretch were noninvasively monitored and used to calculate stiffness in the range of 80-120 mmHg and then to successfully predict graft burst strength. The length of the graft was incrementally shortened during bioreactor culture to maintain circumferential alignment and achieve mechanical anisotropy comparable to native arteries. After 7-9 weeks of bioreactor culture, the fibrin-based grafts were extensively remodeled by the fibroblasts into circumferentially-aligned tubes of collagen and other extracellular matrix with burst pressures in the range of 1400-1600 mmHg and compliance comparable to native arteries. The tissue suture retention force was also suitable for implantation in the rat model and, with poly(lactic acid) sewing rings entrapped at both ends of the graft, also in the ovine model. The strength achieved with a biological scaffold in such a short duration is unprecedented for an engineered artery.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures


References
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480–486. - PubMed
-
- Kannan RY, Salacinski HJ, Butler PE, Hamilton G, Seifalian AM. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res B Appl Biomater. 2005;74(1):570–581. - PubMed
-
- Conte MS. The ideal small arterial substitute: a search for the holy grail? Faseb J. 1998;12(1):43–45. - PubMed
-
- Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006;98(1):25–35. - PubMed
-
- L'Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12(1):47–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

