Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure
- PMID: 20934413
- PMCID: PMC2993873
- DOI: 10.1016/j.brainres.2010.10.009
Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure
Abstract
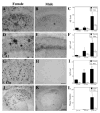
The risk of Alzheimer's disease (AD) is higher in women than in men, a sex difference that likely results from the effects of sex steroid hormones. To investigate this relationship, we first compared progression of β-amyloid (Aβ) pathology in male and female triple transgenic (3xTg-AD) mice. We found that female 3xTg-AD mice exhibit significantly greater Aβ burden and larger behavioral deficits than age-matched males. Next, we evaluated how the organizational effects of sex steroid hormones during postnatal development may affect adult vulnerability to Aβ pathology. We observed that male 3xTg-AD mice demasculinized during early development exhibit significantly increased Aβ accumulation in adulthood. In contrast, female mice defeminized during early development exhibit a more male-like pattern of Aβ pathology in adulthood. Taken together, these results demonstrate significant sex differences in pathology in 3xTg-AD mice and suggest that these differences may be mediated by organizational actions of sex steroid hormones during development.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures

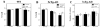


References
-
- Akhmadeev AV, Kalimullina LB. [Neonatal androgenization of female rats during sex differentiation of the brain modifies neuronal organization of the amygdala complex] Ontogenez. 2005;36:64–7. - PubMed
-
- Akhondzadeh S, Rezaei F, Larijani B, Nejatisafa AA, Kashani L, Abbasi SH. Correlation between testosterone, gonadotropins and prolactin and severity of negative symptoms in male patients with chronic schizophrenia. Schizophr Res. 2006;84:405–10. - PubMed
-
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–8. - PubMed
-
- Anahara R, Toyama Y, Mori C. Flutamide induces ultrastructural changes in spermatids and the ectoplasmic specialization between the Sertoli cell and spermatids in mouse testes. Reprod Toxicol. 2004;18:589–96. - PubMed
-
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM, Dartigues J-F, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurology. 1999;53:1992–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

