The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70
- PMID: 20934464
- PMCID: PMC3014454
- DOI: 10.1016/j.bbamcr.2010.08.012
The maximal cytoprotective function of the heat shock protein 27 is dependent on heat shock protein 70
Abstract
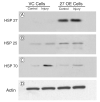
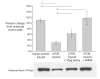
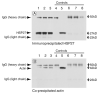
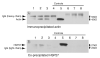
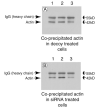
Endogenous heat shock proteins (HSPs) 70 and 25/27 are induced in renal cells by injury from energy depletion. Transfected over-expression of HSPs 70 or 27 (human analogue of HSP25), provide protection against renal cell injury from ATP deprivation. This study examines whether over-expressed HSP27 depends on induction of endogenous HSPs, in particular HSP70, to afford protection against cell injury. LLC-PK1 cells transfected with HSP27 (27OE cells) were injured by ATP depletion for 2h and recovered for 4h in the presence of HSF decoy, HSP70 specific siRNA (siRNA-70) and their respective controls. Injury in the presence of HSF decoy, a synthetic oligonucleotide identical to the heat shock element, the nuclear binding site of HSF, decreased HSP70 induction by 80% without affecting the over-expression of transfected HSP27. The HSP70 stress response was completely ablated in the presence of siRNA-70. Protection against injury, provided by over-expression of HSP27, was reduced by treatment with HSF decoy and abolished by treatment with siRNA-70. Immunoprecipitation studies demonstrated association of HSP27 with actin that was not affected by either treatment with HSF decoy or siRNA. Therefore, HSP27 is dependent on HSP70 to provide its maximal cytoprotective effect, but not for its interaction with actin. This study suggests that, while it has specific action on the cytoskeleton, HSP 25/27 must have coordinated activity with other HSP classes, especially HSP70, to provide the full extent of resistance to injury from energy depletion.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Polyamines mediate glutamine-dependent induction of the intestinal epithelial heat shock response.Am J Physiol Gastrointest Liver Physiol. 2011 Jul;301(1):G181-7. doi: 10.1152/ajpgi.00054.2011. Epub 2011 Apr 21. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21512157 Free PMC article.
-
Heterogeneous nuclear ribonucleoprotein K inhibits heat shock-induced transcriptional activity of heat shock factor 1.J Biol Chem. 2017 Aug 4;292(31):12801-12812. doi: 10.1074/jbc.M117.774992. Epub 2017 Jun 7. J Biol Chem. 2017. PMID: 28592492 Free PMC article.
-
Bicyclol: a novel antihepatitis drug with hepatic heat shock protein 27/70-inducing activity and cytoprotective effects in mice.Cell Stress Chaperones. 2008 Sep;13(3):347-55. doi: 10.1007/s12192-008-0034-4. Epub 2008 Apr 8. Cell Stress Chaperones. 2008. PMID: 18392951 Free PMC article.
-
HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review).Int J Oncol. 2014 Jul;45(1):18-30. doi: 10.3892/ijo.2014.2399. Epub 2014 Apr 25. Int J Oncol. 2014. PMID: 24789222 Review.
-
Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties.Cell Cycle. 2006 Nov;5(22):2592-601. doi: 10.4161/cc.5.22.3448. Epub 2006 Nov 15. Cell Cycle. 2006. PMID: 17106261 Review.
Cited by
-
Mice with an absent stress response are protected against ischemic renal injury.Kidney Int. 2014 Sep;86(3):515-24. doi: 10.1038/ki.2014.73. Epub 2014 May 7. Kidney Int. 2014. PMID: 24805105 Free PMC article.
-
Heat shock proteins and kidney disease: perspectives of HSP therapy.Cell Stress Chaperones. 2017 May;22(3):319-343. doi: 10.1007/s12192-017-0790-0. Epub 2017 Apr 13. Cell Stress Chaperones. 2017. PMID: 28409327 Free PMC article. Review.
-
Acute pancreatitis: the stress factor.World J Gastroenterol. 2014 May 21;20(19):5801-7. doi: 10.3748/wjg.v20.i19.5801. World J Gastroenterol. 2014. PMID: 24914340 Free PMC article. Review.
-
Heat shock proteins in the kidney.Pediatr Nephrol. 2016 Oct;31(10):1561-70. doi: 10.1007/s00467-015-3297-x. Epub 2016 Feb 25. Pediatr Nephrol. 2016. PMID: 26913726 Review.
-
Analysis of protein expression in periodontal pocket tissue: a preliminary study.Proteome Sci. 2015 Dec 30;13:33. doi: 10.1186/s12953-015-0089-y. eCollection 2015. Proteome Sci. 2015. PMID: 26719749 Free PMC article.
References
-
- Molitoris BA, Hoilien CA, Dahl R, Ahnen DJ, Wilson PD, Kim J. Characterization of ischemia-induced loss of epithelial polarity. J. Membr. Biol. 1988;106:233–242. - PubMed
-
- Bagshaw SM, George C, Gibney RT, Bellomo R. A multi-center evaluation of early acute kidney injury in critically ill trauma patients. Ren. Fail. 2008;30:581–589. - PubMed
-
- Atkinson SJ, Hosford MA, Molitoris BA. Mechanism of actin polymerization in cellular ATP depletion. J. Biol. Chem. 2004;279:5194–5199. - PubMed
-
- Molitoris BA, Dahl R, Geerdes A. Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am. J. Physiol. 1992;263:F488–F495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

