Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms
- PMID: 20935178
- PMCID: PMC2996015
- DOI: 10.1104/pp.110.166454
Evidence for the existence of one antenna-associated, lipid-dissolved and two protein-bound pools of diadinoxanthin cycle pigments in diatoms
Abstract
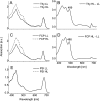
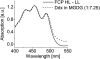
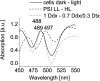
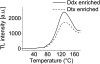
We studied the localization of diadinoxanthin cycle pigments in the diatoms Cyclotella meneghiniana and Phaeodactylum tricornutum. Isolation of pigment protein complexes revealed that the majority of high-light-synthesized diadinoxanthin and diatoxanthin is associated with the fucoxanthin chlorophyll protein (FCP) complexes. The characterization of intact cells, thylakoid membranes, and pigment protein complexes by absorption and low-temperature fluorescence spectroscopy showed that the FCPs contain certain amounts of protein-bound diadinoxanthin cycle pigments, which are not significantly different in high-light and low-light cultures. The largest part of high-light-formed diadinoxanthin cycle pigments, however, is not bound to antenna apoproteins but located in a lipid shield around the FCPs, which is copurified with the complexes. This lipid shield is primarily composed of the thylakoid membrane lipid monogalactosyldiacylglycerol. We also show that the photosystem I (PSI) fraction contains a tightly connected FCP complex that is enriched in protein-bound diadinoxanthin cycle pigments. The peripheral FCP and the FCP associated with PSI are composed of different apoproteins. Tandem mass spectrometry analysis revealed that the peripheral FCP is composed mainly of the light-harvesting complex protein Lhcf and also significant amounts of Lhcr. The PSI fraction, on the other hand, shows an enrichment of Lhcr proteins, which are thus responsible for the diadinoxanthin cycle pigment binding. The existence of lipid-dissolved and protein-bound diadinoxanthin cycle pigments in the peripheral antenna and in PSI is discussed with respect to different specific functions of the xanthophylls.
Figures



Similar articles
-
The diadinoxanthin diatoxanthin cycle induces structural rearrangements of the isolated FCP antenna complexes of the pennate diatom Phaeodactylum tricornutum.Plant Physiol Biochem. 2015 Nov;96:364-76. doi: 10.1016/j.plaphy.2015.09.002. Epub 2015 Sep 5. Plant Physiol Biochem. 2015. PMID: 26368016
-
Pre-purification of diatom pigment protein complexes provides insight into the heterogeneity of FCP complexes.BMC Plant Biol. 2020 Oct 6;20(1):456. doi: 10.1186/s12870-020-02668-x. BMC Plant Biol. 2020. PMID: 33023504 Free PMC article.
-
Isolation of fucoxanthin chlorophyll protein complexes of the centric diatom Thalassiosira pseudonana associated with the xanthophyll cycle enzyme diadinoxanthin de-epoxidase.IUBMB Life. 2023 Jan;75(1):66-76. doi: 10.1002/iub.2650. Epub 2022 May 26. IUBMB Life. 2023. PMID: 35557488
-
Structural features of the diatom photosystem II-light-harvesting antenna complex.FEBS J. 2020 Jun;287(11):2191-2200. doi: 10.1111/febs.15183. Epub 2020 Jan 7. FEBS J. 2020. PMID: 31854056 Review.
-
Photosynthetic Pigments in Diatoms.Mar Drugs. 2015 Sep 16;13(9):5847-81. doi: 10.3390/md13095847. Mar Drugs. 2015. PMID: 26389924 Free PMC article. Review.
Cited by
-
Phylogenetic viewpoints on regulation of light harvesting and electron transport in eukaryotic photosynthetic organisms.Planta. 2013 Feb;237(2):399-412. doi: 10.1007/s00425-012-1744-5. Epub 2012 Sep 13. Planta. 2013. PMID: 22971817 Review.
-
Dynamic Changes between Two LHCX-Related Energy Quenching Sites Control Diatom Photoacclimation.Plant Physiol. 2018 Jul;177(3):953-965. doi: 10.1104/pp.18.00448. Epub 2018 May 17. Plant Physiol. 2018. PMID: 29773581 Free PMC article.
-
Unique photosynthetic strategies employed by closely related Breviolum minutum strains under rapid short-term cumulative heat stress.J Exp Bot. 2024 Jul 10;75(13):4005-4023. doi: 10.1093/jxb/erae170. J Exp Bot. 2024. PMID: 38636949 Free PMC article.
-
Molecular and photosynthetic responses to prolonged darkness and subsequent acclimation to re-illumination in the diatom Phaeodactylum tricornutum.PLoS One. 2013;8(3):e58722. doi: 10.1371/journal.pone.0058722. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520530 Free PMC article.
-
Rapid regulation of excitation energy in two pennate diatoms from contrasting light climates.Photosynth Res. 2018 Nov;138(2):149-165. doi: 10.1007/s11120-018-0558-0. Epub 2018 Jul 14. Photosynth Res. 2018. PMID: 30008155 Free PMC article.
References
-
- Amunts A, Nelson N. (2008) Functional organization of a plant photosystem I: evolution of a highly efficient photochemical machine. Plant Physiol Biochem 46: 228–237 - PubMed
-
- Anderson R, Kates M, Volcani BE. (1978) Identification of the sulfolipids in the non-photosynthetic diatom Nitzschia alba. Biochim Biophys Acta 528: 89–106 - PubMed
-
- Bassi R, Pineau B, Dainese P, Marquardt J. (1993) Carotenoid-binding proteins of photosystem II. Eur J Biochem 212: 297–303 - PubMed
-
- Beer A, Gundermann K, Beckmann J, Büchel C. (2006) Subunit composition and pigmentation of fucoxanthin-chlorophyll proteins in diatoms: evidence for a subunit involved in diadinoxanthin and diatoxanthin binding. Biochemistry 45: 13046–13053 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

