Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium
- PMID: 20935191
- PMCID: PMC3175555
- DOI: 10.1165/rcmb.2010-0253OC
Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium
Abstract
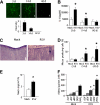
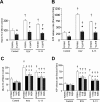
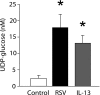
Adenosine triphosphate (ATP) and its metabolite adenosine regulate airway mucociliary clearance via activation of purinoceptors. In this study, we investigated the contribution of goblet cells to airway epithelial ATP release. Primary human bronchial epithelial (HBE) cultures, typically dominated by ciliated cells, were induced to develop goblet cell metaplasia by infection with respiratory syncytial virus (RSV) or treatment with IL-13. Under resting conditions, goblet-cell metaplastic cultures displayed enhanced mucin secretion accompanied by increased rates of ATP release and mucosal surface adenosine accumulation as compared with nonmetaplastic control HBE cultures. Intracellular calcium chelation [1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid tetraacetoxymethyl ester] or disruption of the secretory pathways (nocodazole, brefeldin A, and N-ethylmaleimide) decreased mucin secretion and ATP release in goblet-cell metaplastic HBE cultures. Conversely, stimuli that triggered calcium-regulated mucin secretion (e.g., ionomycin or UTP) increased luminal ATP release and adenyl purine accumulation in control and goblet-cell metaplastic HBE cultures. Goblet cell-associated ATP release was not blocked by the connexin/pannexin hemichannel inhibitor carbenoxolone, suggesting direct nucleotide release from goblet cell vesicles rather than the hemichannel insertion. Collectively, our data demonstrate that nucleotide release is increased by goblet cell metaplasia, reflecting, at least in part, a mechanism tightly associated with goblet cell mucin secretion. Increased goblet cell nucleotide release and resultant adenosine accumulation provide compensatory mechanisms to hydrate mucins by paracrine stimulation of ciliated cell ion and water secretion and maintain mucociliary clearance, and to modulate inflammatory responses.
Figures



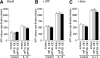
Similar articles
-
Secretory phospholipases A2 are secreted from ciliated cells and increase mucin and eicosanoid secretion from goblet cells.Chest. 2015 Jun;147(6):1599-1609. doi: 10.1378/chest.14-0258. Chest. 2015. PMID: 25429648 Free PMC article.
-
Regulation of mucin secretion from human bronchial epithelial cells grown in murine hosted xenografts.Am J Physiol Lung Cell Mol Physiol. 2003 Jun;284(6):L945-54. doi: 10.1152/ajplung.00410.2002. Epub 2003 Jan 17. Am J Physiol Lung Cell Mol Physiol. 2003. PMID: 12533443
-
Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides.J Physiol. 2010 Jun 15;588(Pt 12):2255-67. doi: 10.1113/jphysiol.2009.186643. Epub 2010 Apr 26. J Physiol. 2010. PMID: 20421285 Free PMC article.
-
Regulation of mucin secretion from in vitro cellular models.Novartis Found Symp. 2002;248:113-25; discussion 125-31, 277-82. Novartis Found Symp. 2002. PMID: 12568491 Review.
-
Regulated airway goblet cell mucin secretion.Annu Rev Physiol. 2008;70:487-512. doi: 10.1146/annurev.physiol.70.113006.100638. Annu Rev Physiol. 2008. PMID: 17988208 Review.
Cited by
-
Extracellular ATP is involved in dsRNA-induced MUC5AC production via P2Y2R in human airway epithelium.Respir Res. 2016 Sep 27;17(1):121. doi: 10.1186/s12931-016-0438-0. Respir Res. 2016. PMID: 27677339 Free PMC article.
-
A sodium binding system alleviates acute salt stress during seawater acclimation in eels.Zoological Lett. 2017 Dec 12;3:22. doi: 10.1186/s40851-017-0081-8. eCollection 2017. Zoological Lett. 2017. PMID: 29255617 Free PMC article.
-
Targeting the P2Y13 Receptor Suppresses IL-33 and HMGB1 Release and Ameliorates Experimental Asthma.Am J Respir Crit Care Med. 2022 Feb 1;205(3):300-312. doi: 10.1164/rccm.202009-3686OC. Am J Respir Crit Care Med. 2022. PMID: 34860143 Free PMC article.
-
Upregulation of TMEM16A Protein in Bronchial Epithelial Cells by Bacterial Pyocyanin.PLoS One. 2015 Jun 29;10(6):e0131775. doi: 10.1371/journal.pone.0131775. eCollection 2015. PLoS One. 2015. PMID: 26121472 Free PMC article.
-
Abnormal Airway Mucus Secretion Induced by Virus Infection.Front Immunol. 2021 Sep 28;12:701443. doi: 10.3389/fimmu.2021.701443. eCollection 2021. Front Immunol. 2021. PMID: 34650550 Free PMC article. Review.
References
-
- Zsembery A, Fortenberry JA, Liang L, Bebok Z, Tucker TA, Boyce AT, Braunstein GM, Welty E, Bell PD, Sorscher EJ, et al. Extracellular zinc and ATP restore chloride secretion across cystic fibrosis airway epithelia by triggering calcium entry. J Biol Chem 2004;279:10720–10729. - PubMed
-
- Zsembery A, Boyce AT, Liang L, Peti-Peterdi J, Bell PD, Schwiebert EM. Sustained calcium entry through p2x nucleotide receptor channels in human airway epithelial cells. J Biol Chem 2003;278:13398–13408. - PubMed
-
- Verdugo P, Deyrup-Olsen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: I. The role of intragranular charge shielding. J Dent Res 1987;66:506–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

