Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche
- PMID: 20935204
- PMCID: PMC2975552
- DOI: 10.4049/jimmunol.1002042
Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche
Abstract
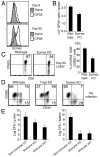
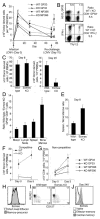
CD8(+) T cells responding to intracellular infection give rise to cellular progeny that become terminally differentiated effector cells and self-renewing memory cells. T-bet and eomesodermin (Eomes) are key transcription factors of cytotoxic lymphocyte lineages. We show in this study that CD8(+) T cells lacking Eomes compete poorly in contributing to the pool of Ag-specific central memory cells. Eomes-deficient CD8(+) T cells undergo primary clonal expansion but are defective in long-term survival, populating the bone marrow niche and re-expanding postrechallenge. The phenotype of Eomes-deficient CD8(+) T cells supports the hypothesis that T-bet and Eomes can act redundantly to induce effector functions, but can also act to reciprocally promote terminal differentiation versus self-renewal of Ag-specific memory cells.
Figures

References
-
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. - PubMed
-
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. - PubMed
-
- Becker TC, Coley SM, Wherry EJ, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J Immunol. 2005;174:1269–1273. - PubMed
-
- Letsch A, Knoedler M, Na IK, Kern F, Asemissen AM, Keilholz U, Loesch M, Thiel E, Volk HD, Scheibenbogen C. CMV-specific central memory T cells reside in bone marrow. Eur J Immunol. 2007;37:3063–3068. - PubMed
-
- Mazo IB, Honczarenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007324/AI/NIAID NIH HHS/United States
- R56 AI076458/AI/NIAID NIH HHS/United States
- T32 AI055428/AI/NIAID NIH HHS/United States
- T32 CA009140/CA/NCI NIH HHS/United States
- R01 AI042370/AI/NIAID NIH HHS/United States
- CA076931/CA/NCI NIH HHS/United States
- R01 AI061699/AI/NIAID NIH HHS/United States
- R01 AI071309/AI/NIAID NIH HHS/United States
- AI007324/AI/NIAID NIH HHS/United States
- K12 CA076931/CA/NCI NIH HHS/United States
- K08 HL093027/HL/NHLBI NIH HHS/United States
- AI076458/AI/NIAID NIH HHS/United States
- AI055428/AI/NIAID NIH HHS/United States
- CA09140/CA/NCI NIH HHS/United States
- AI061699/AI/NIAID NIH HHS/United States
- R01 AI076458/AI/NIAID NIH HHS/United States
- AI071309/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

