Blockade of the ubiquitin protease UBP43 destabilizes transcription factor PML/RARα and inhibits the growth of acute promyelocytic leukemia
- PMID: 20935222
- PMCID: PMC2999664
- DOI: 10.1158/0008-5472.CAN-10-1100
Blockade of the ubiquitin protease UBP43 destabilizes transcription factor PML/RARα and inhibits the growth of acute promyelocytic leukemia
Abstract
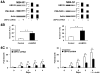
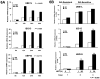
More effective treatments for acute promyelocytic leukemia (APL) are needed. APL cell treatment with all-trans-retinoic acid (RA) degrades the chimeric, dominant-negative-acting transcription factor promyelocytic leukemia gene (PML)/RARα, which is generated in APL by chromosomal translocation. The E1-like ubiquitin-activating enzyme (UBE1L) associates with interferon-stimulated gene ISG15 that binds and represses PML/RARα protein. Ubiquitin protease UBP43/USP18 removes ISG15 from conjugated proteins. In this study, we explored how RA regulates UBP43 expression and the effects of UBP43 on PML/RARα stability and APL growth, apoptosis, or differentiation. RA treatment induced UBE1L, ISG15, and UBP43 expression in RA-sensitive but not RA-resistant APL cells. Similar in vivo findings were obtained in a transgenic mouse model of transplantable APL, and in the RA response of leukemic cells harvested directly from APL patients. UBP43 knockdown repressed PML/RARα protein levels and inhibited RA-sensitive or RA-resistant cell growth by destabilizing the PML domain of PML/RARα. This inhibitory effect promoted apoptosis but did not affect the RA differentiation response in these APL cells. In contrast, elevation of UBP43 expression stabilized PML/RARα protein and inhibited apoptosis. Taken together, our findings define the ubiquitin protease UBP43 as a novel candidate drug target for APL treatment.
Conflict of interest statement
No potential conflicts of interest exist.
Figures




References
-
- Larson RA, Kondo K, Vardiman JW, Butler AE, Golomb HM, Rowley JD. Evidence for a 15;17 translocation in every patient with acute promyelocytic leukemia. Am J Med. 1984;76:827–41. - PubMed
-
- de Thé H, Lavau C, Marchio A, Chomienne C, Degos L, Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991;66:675–84. - PubMed
-
- Kakizuka A, Miller WH, Jr, Umesono K, Warrell RP, Jr, Frankel SR, Murty VV, Dmitrovsky E, Evans RM. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991;66:663–74. - PubMed
-
- Pitha-Rowe I, Petty WJ, Kitareewan S, Dmitrovsky E. Retinoid target genes in acute promyelocytic leukemia. Leukemia. 2003;17:1723–30. - PubMed
-
- Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene. 2003;22:7305–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

