Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells
- PMID: 20935229
- PMCID: PMC3023291
- DOI: 10.1152/ajplung.00142.2010
Regulation of endogenous ENaC functional expression by CFTR and ΔF508-CFTR in airway epithelial cells
Abstract
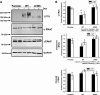
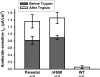
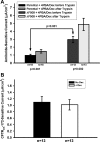
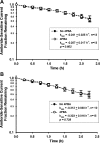
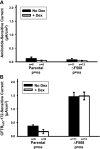
The functional expression of the epithelial sodium channel (ENaC) appears elevated in cystic fibrosis (CF) airway epithelia, but the mechanism by which this occurs is not clear. We tested the hypothesis that the cystic fibrosis transmembrane conductance regulator (CFTR) alters the trafficking of endogenously expressed human ENaC in the CFBE41o⁻ model of CF bronchial epithelia. Functional expression of ENaC, as defined by amiloride-inhibited short-circuit current (I(sc)) in Ussing chambers, was absent under control conditions but present in CFBE41o⁻ parental and ΔF508-CFTR-overexpressing cells after treatment with 1 μM dexamethasone (Dex) for 24 h. The effect of Dex was mimicked by incubation with the glucocorticoid hydrocortisone but not with the mineralocorticoid aldosterone. Application of trypsin to the apical surface to activate uncleaved, "near-silent" ENaC caused an additional increase in amiloride-sensitive I(sc) in the Dex-treated cells and was without effect in the control cells, suggesting that Dex increased ENaC cell surface expression. In contrast, Dex treatment did not stimulate amiloride-sensitive I(sc) in CFBE41o⁻ cells that stably express wild-type (wt) CFTR. CFBE41o⁻ wt cells also had reduced expression of α- and γ-ENaC compared with parental and ΔF508-CFTR-overexpressing cells. Furthermore, application of trypsin to the apical surface of Dex-treated CFBE41o⁻ wt cells did not stimulate amiloride-sensitive I(sc), suggesting that ENaC remained absent from the surface of these cells even after Dex treatment. We also tested the effect of trafficking-corrected ΔF508-CFTR on ENaC functional expression. Incubation with 1 mM 4-phenylbutyrate synergistically increased Dex-induced ENaC functional expression in ΔF508-CFTR-overexpressing cells. These data support the hypothesis that wt CFTR can regulate the whole cell, functional, and surface expression of endogenous ENaC in airway epithelial cells and that absence of this regulation may foster ENaC hyperactivity in CF airway epithelia.
Figures



References
-
- Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol 283: L329–L335, 2002 - PubMed
-
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem 276: 6621–6630, 2001 - PubMed
-
- Brown SG, Gallacher M, Olver RE, Wilson SM. The regulation of selective and nonselective Na+ conductances in H441 human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L942–L954, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

