Inhibition of NADPH oxidase by glucosylceramide confers chemoresistance
- PMID: 20935456
- PMCID: PMC3047104
- DOI: 10.4161/cbt.10.11.13438
Inhibition of NADPH oxidase by glucosylceramide confers chemoresistance
Abstract
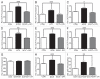
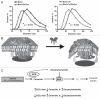
The bioactive sphingolipid ceramide induces oxidative stress by disrupting mitochondrial function and stimulating NADPH oxidase (NOX) activity, both implicated in cell death mechanisms. Many anticancer chemotherapeutics (anthracyclines, Vinca alkaloids, paclitaxel, and fenretinide), as well as physiological stimuli such as tumor necrosis factor α (TNFα), stimulate ceramide accumulation and increase oxidative stress in malignant cells. Consequently, ceramide metabolism in malignant cells and, in particular the up-regulation of glucosylceramide synthase (GCS), has gained considerable interest in contributing to chemoresistance. We hypothesized that increases in GCS activity and thus glucosylceramide, the product of GCS activity, represents an important resistance mechanism in glioblastoma. In our study, we determined that increased GCS activity effectively blocked reactive oxygen species formation by NOX. We further showed, in both glioblastoma and neuroblastoma cells that glucosylceramide directly interfered with NOX assembly, hence delineating a direct resistance mechanism. Collectively, our findings indicated that pharmacological or molecular targeting of GCS, using non-toxic nanoliposome delivery systems, successfully augmented NOX activity, and improved the efficacy of known chemotherapeutic agents.
Figures



References
-
- Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: Relationship with oxidative stress. Free Radic Biol Med. 2001;31:717–728. - PubMed
-
- Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. - PubMed
-
- Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, et al. Ceramides and other bioactive sphingolipid backbones in health and disease: Lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta. 2006;1758:1864–1884. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
-
- Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: Roles of NAD(P)H oxidase, oxidant stress and ceramide. Diabetes. 2005;54:1838–1845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
