Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells
- PMID: 20935507
- PMCID: PMC3047752
- DOI: 10.4161/cc.9.19.13139
Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells
Abstract
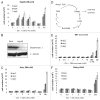
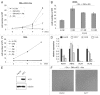
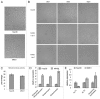
Glutaminolysis and Warburg effect are the two most noticeable metabolic features of tumor cells whereas their biological significance in cell proliferation remains elusive. A widely accepted current hypothesis is that tumor cells use glutamine as a preferred carbon source for energy and reducing power, which has been used to explain both glutaminolysis and the Warburg effect. Here we provide evidence to show that supplying nitrogen, not the carbon skeleton, underlies the major biological importance of glutaminolysis for proliferating cells. We show alternative nitrogen supplying mechanisms rescue cell proliferation in glutamine-free media. Particularly, we show that ammonia is sufficient to maintain a long-term survival and proliferation of Hep3B in glutamine-free media. We also observed that nitrogen source restriction repressed carbon metabolic pathways including glucose utilization. Based on these new observations and metabolic pathways well established in published literature, we propose an alternative model that cellular demand for glutamate as a key molecule in nitrogen anabolism is the driving force of glutaminolysis in proliferating cells. Our model suggests that the Warburg effect may be a metabolic consequence secondary to the nitrogen anabolism.
Figures
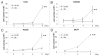




References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Klimberg VS, McClellan JL, Organ CH., Jr Honorary Lectureship. Glutamine, cancer and its therapy. Am J Surg. 1996;172:418–424. - PubMed
-
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. - PubMed
-
- Medina MA. Glutamine and cancer. J Nutr. 2001;131:2539–2542. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
