The emerging role of autophagy in the pathophysiology of diabetes mellitus
- PMID: 20935516
- PMCID: PMC3359481
- DOI: 10.4161/auto.7.1.13044
The emerging role of autophagy in the pathophysiology of diabetes mellitus
Abstract
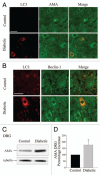
An emerging body of evidence supports a role for autophagy in the pathophysiology of type 1 and type 2 diabetes mellitus. Persistent high concentrations of glucose lead to imbalances in the antioxidant capacity within the cell resulting in oxidative stress-mediated injury in both disorders. An anticipated consequence of impaired autophagy is the accumulation of dysfunctional organelles such as mitochondria within the cell. Mitochondria are the primary site of the production of reactive oxygen species (ROS), and an imbalance in ROS production relative to the cytoprotective action of autophagy may lead to the accumulation of ROS. Impaired mitochondrial function associated with increased ROS levels have been proposed as mechanisms contributing to insulin resistance. In this article we review and interpret the literature that implicates a role for autophagy in the pathophysiology of type 1 and type 2 diabetes mellitus as it applies to β-cell dysfunction, and more broadly to organ systems involved in complications of diabetes including the cardiovascular, renal and nervous systems.
Figures


References
-
- Kim EH, Sohn S, Kwon HJ, Kim SU, Kim MJ, Lee SJ, Choi KS. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent autophagic cell death in malignant glioma cells. Cancer Res. 2007;67:6314–6324. - PubMed
-
- Park KJ, Lee SH, Kim TI, Lee HW, Lee CH, Kim EH, et al. 3: A human scfv antibody against trail receptor 2 induces autophagic cell death in both trail-sensitive and trail-resistant cancer cells. Cancer Res. 2008;67:7317–7334. - PubMed
-
- Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, et al. Essential roles of atg5 and FADD in autophagic cell death: Dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2007;280:20722–20729. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
