IL-37 is a fundamental inhibitor of innate immunity
- PMID: 20935647
- PMCID: PMC3537119
- DOI: 10.1038/ni.1944
IL-37 is a fundamental inhibitor of innate immunity
Abstract
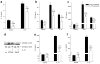
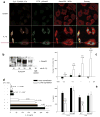
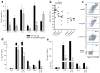
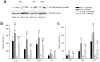
The function of interleukin 37 (IL-37; formerly IL-1 family member 7) has remained elusive. Expression of IL-37 in macrophages or epithelial cells almost completely suppressed production of pro-inflammatory cytokines, whereas the abundance of these cytokines increased with silencing of endogenous IL-37 in human blood cells. Anti-inflammatory cytokines were unaffected. Mice with transgenic expression of IL-37 were protected from lipopolysaccharide-induced shock, and showed markedly improved lung and kidney function and reduced liver damage after treatment with lipopolysaccharide. Transgenic mice had lower concentrations of circulating and tissue cytokines (72-95% less) than wild-type mice and showed less dendritic cell activation. IL-37 interacted intracellularly with Smad3 and IL-37-expressing cells and transgenic mice showed less cytokine suppression when endogenous Smad3 was depleted. IL-37 thus emerges as a natural suppressor of innate inflammatory and immune responses.
Conflict of interest statement
The authors have no conflicting financial interests.
Figures




References
-
- Schmitz J, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
-
- Dunn E, Sims JE, Nicklin MJ, O'Neill LA. Annotating genes with potential roles in the immune system: six new members of the IL-1 family. Trends Immunol. 2001;22:533–536. - PubMed
-
- Pan G, et al. IL-1H, an interleukin 1-related protein that binds IL-18 receptor/IL-1Rrp. Cytokine. 2001;13:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

