Porphyrins as Molecular Electronic Components of Functional Devices
- PMID: 20936084
- PMCID: PMC2950646
- DOI: 10.1016/j.ccr.2010.05.014
Porphyrins as Molecular Electronic Components of Functional Devices
Abstract
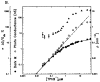
The proposal that molecules can perform electronic functions in devices such as diodes, rectifiers, wires, capacitors, or serve as functional materials for electronic or magnetic memory, has stimulated intense research across physics, chemistry, and engineering for over 35 years. Because biology uses porphyrins and metalloporphyrins as catalysts, small molecule transporters, electrical conduits, and energy transducers in photosynthesis, porphyrins are an obvious class of molecules to investigate for molecular electronic functions. Of the numerous kinds of molecules under investigation for molecular electronics applications, porphyrins and their related macrocycles are of particular interest because they are robust and their electronic properties can be tuned by chelation of a metal ion and substitution on the macrocycle. The other porphyrinoids have equally variable and adjustable photophysical properties, thus photonic applications are potentiated. At least in the near term, realistic architectures for molecular electronics will require self-organization or nanoprinting on surfaces. This review concentrates on self-organized porphyrinoids as components of working electronic devices on electronically active substrates with particular emphasis on the effect of surface, molecular design, molecular orientation and matrix on the detailed electronic properties of single molecules.
Figures







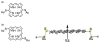




References
-
- Abruña HD, Ratner MA, Zee RDv, González CA, Kagan CR, Stewart DR, Walker AV, Batteas JD, Chidsey CED, Seideman T. Ballston, Virginia: 2007. National Science Foundation pp.
- Mirkin CA, Ratner MA. Annual Rev Phys Chem. 1992;43:719.
- Annal New York Acad Sci. 2002;960
-
- Hille B. Ion Channels of Excitable Membranes. 3. Sinauer Associates; Sunderland: 2001.
-
- Hong FT. Biosensors and Biocomputers. Springer; New York: 1990. Molecular Electronics.
-
- Stoddart JF. Chem Soc Rev. 2009;38:1802. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
